Abstract
The nuclear hormone receptors (NRs) form a superfamily of transcription factors unified by conserved protein structure and mode of function. While most members of this superfamily are activated by ligands, such as thyroid hormones, steroids, vitamin D or retinoic acid, other NRs are called orphan receptors because they have no known ligand. NR-dependent signaling is crucial for vertebrate development with the majority of receptors being expressed in the developing embryo. Due to massive gene duplications during vertebrate diversification, there are usually more NRs in vertebrates than in invertebrates. In this study, we examine the evolutionary diversification of the NR superfamily and of NR-dependent signaling in chordates (vertebrates, tunicates, and amphioxus). We take advantage of the unique features of the genome of the invertebrate amphioxus, which is characterized by a vertebrate-like gene content without having undergone massive duplications, to assess the NR signaling complement (NRs and NR coregulators) of the ancestral chordate. We find 33 NRs in amphioxus, which are more NRs than originally anticipated. This increase is mainly due to an amphioxus-specific duplication of genes encoding receptors of the NR1H group. In addition, there are three heterologous NRs in amphioxus that could not be placed within the framework of the NR superfamily. Apart from these exceptions, there is usually one amphioxus NR or NR signaling coregulator for each paralogous group of two, three, or four human receptors suggesting that the ancestral chordate had a set of 22 different NRs plus one copy of each NR coregulator.



Similar content being viewed by others
References
Allen AK, Spradling AC (2008) The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 135:311–321
Baker ME (2003) Evolution of adrenal and sex steroid action in vertebrates: a ligand-based mechanism for complexity. Bioessays 25:396–400
Bardet PL, Schubert M, Horard B, Holland LZ, Laudet V, Holland ND, Vanacker JM (2005) Expression of estrogen-receptor related receptors in amphioxus and zebrafish: implications for the evolution of posterior brain segmentation at the invertebrate-to-vertebrate transition. Evol Dev 7:223–233
Bastien J, Rochette-Egly C (2004) Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 328:1–16
Benoit G, Cooney A, Giguere V, Ingraham H, Lazar M, Muscat G, Perlmann T, Renaud JP, Schwabe J, Sladek F, Tsai MJ, Laudet V (2006) International Union of Pharmacology. LXVI. Orphan nuclear receptors. Pharmacol Rev 58:798–836
Bertrand S, Brunet FG, Escriva H, Parmentier G, Laudet V, Robinson-Rechavi M (2004) Evolutionary genomics of nuclear receptors: from twenty-five ancestral genes to derived endocrine systems. Mol Biol Evol 21:1923–1937
Bertrand S, Thisse B, Tavares R, Sachs L, Chaumot A, Bardet PL, Escriva H, Duffraisse M, Marchand O, Safi R, Thisse C, Laudet V (2007) Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet 3:e188
Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE (2002) Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110:93–105
Bledsoe RK, Madauss KP, Holt JA, Apolito CJ, Lambert MH, Pearce KH, Stanley TB, Stewart EL, Trump RP, Willson TM, Williams SP (2005) A ligand-mediated hydrogen bond network required for the activation of the mineralocorticoid receptor. J Biol Chem 280:31283–31293
Bonneton F, Chaumot A, Laudet V (2008) Annotation of Tribolium nuclear receptors reveals an increase in evolutionary rate of a network controlling the ecdysone cascade. Insect Biochem Mol Biol 38:416–429
Bridgham JT, Carroll SM, Thornton JW (2006) Evolution of hormone-receptor complexity by molecular exploitation. Science 312:97–101
Campo-Paysaa F, Marlétaz F, Laudet V, Schubert M (2008) Retinoic acid signaling in development: tissue-specific functions and evolutionary origins. Genesis, in press
Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JÅ (2006) International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 58:773–781
Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang HG, Awazu S, Azumi K, Boore J, Branno M, Chin-Bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee BI, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, Shin-i T, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS (2002) The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298:2157–2167
Dehal P, Boore JL (2005) Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3:e314
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Escriva H, Safi R, Hänni C, Langlois MC, Saumitou-Laprade P, Stehelin D, Capron A, Pierce R, Laudet V (1997) Ligand binding was acquired during evolution of nuclear receptors. Proc Natl Acad Sci USA 94:6803–6808
Escriva H, Delaunay F, Laudet V (2000) Ligand binding and nuclear receptor evolution. Bioessays 22:717–727
Escriva H, Holland ND, Gronemeyer H, Laudet V, Holland LZ (2002) The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development 129:2905–2916
Escriva H, Bertrand S, Germain P, Robinson-Rechavi M, Umbhauer M, Cartry J, Duffraisse M, Holland LZ, Gronemeyer H, Laudet V (2006) Neofunctionalization in vertebrates: the example of retinoic acid receptors. PLoS Genet 2:e102
Flamant F, Baxter JD, Forrest D, Refetoff S, Samuels H, Scanlan TS, Vennström B, Samarut J (2006) International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev 58:705–711
Galtier N, Gouy M, Gautier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
Garcia-Fernàndez J, D’Aniello S, Escriva H (2007) Organizing chordates with an organizer. Bioessays 29:619–624
Germain P, Chambon P, Eichele G, Evans GM, Lazar MA, Leid M, de Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H (2006a) International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev 58:712–725
Germain P, Chambon P, Eichele G, Evans GM, Lazar MA, Leid M, de Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H (2006b) International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev 58:760–772
Germain P, Staels B, Dacquet C, Spedding M, Laudet V (2006c) Overview of nomenclature of nuclear receptors. Pharmacol Rev 58:685–704
Glardon S, Holland LZ, Gehring WJ, Holland ND (1998) Isolation and developmental expression of the amphioxus Pax-6 gene (AmphiPax-6): insights into eye and photoreceptor evolution. Development 125:2701–2710
Goodson M, Jonas BA, Privalsky MA (2005) Corepressors: custom tailoring and alterations while you wait. Nucl Recept Signal 3:e003
Gronemeyer H, Gustafsson JÅ, Laudet V (2004) Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 3:950–964
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Holland LZ, Albalat R, Azumi K, Benito-Gutiérrez E, Blow MJ, Bronner-Fraser M, Brunet F, Butts T, Candiani S, Dishaw LJ, Ferrier DEK, Garcia-Fernàndez J, Gibson-Brown JJ, Gissi C, Godzik A, Hallböök F, Hirose D, Hosomichi K, Ikuta T, Inoko H, Kasahara M, Kasamatsu J, Kawashima T, Kimura A, Kobayashi M, Kozmik Z, Kubokawa K, Laudet V, Litman GW, McHardy AC, Meulemans D, Nonaka M, Olinski RP, Pancer Z, Pennacchio LA, Pestarino M, Rast JP, Rigoutsos I, Robinson-Rechavi M, Roch G, Saiga H, Sasakura Y, Satake M, Satou Y, Schubert M, Sherwood N, Shiina T, Takatori N, Tello J, Vopalensky P, Wada S, Xu A, Ye Y, Yoshida K, Yoshizaki F, Yu JK, Zhang Q, Zmasek CM, de Jong PJ, Osoegawa K, Putnam NH, Rokhsar DS, Satoh N, Holland PWH (2008) The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res 18:1100–1111
Holland PWH, Garcia-Fernàndez J, Williams NA, Sidow A (1994) Gene duplications and the origins of vertebrate development. Development Suppl 1994:125–133
Horard B, Castet A, Bardet PL, Laudet V, Cavailles V, Vanacker JM (2004) Dimerization is required for transactivation by estrogen-receptor-related (ERR) orphan receptors: evidence from amphioxus ERR. J Mol Endocrinol 33:493–509
Howard-Ashby M, Materna SC, Brown CT, Chen L, Cameron RA, Davidson EH (2006) Gene families encoding transcription factors expressed in early development of Strongylocentrotus purpuratus. Dev Biol 300:90–107
Hu X, Lazar MA (2000) Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab 11:6–10
King-Jones K, Thummel CS (2005) Nuclear receptors—a perspective from Drosophila. Nat Rev Genet 6:311–323
Lacalli TC (2001) New perspectives on the evolution of protochordate sensory and locomotory systems, and the origin of brains and heads. Philos Trans R Soc Lond B 356:1565–1572
Langlois MC, Vanacker JM, Holland ND, Escriva H, Queva C, Laudet V, Holland LZ (2000) Amphicoup-TF, a nuclear orphan receptor of the lancelet Branchiostoma floridae, is implicated in retinoic acid signalling pathways. Dev Genes Evol 210:471–482
Laudet V, Gronemeyer H (2002) The nuclear receptor facts book. Academic Press, San Diego
Lonard DM, O’Malley BW (2006) The expanding cosmos of nuclear receptor coactivators. Cell 125:411–414
Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA (2006) International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev 58:782–797
Marlétaz F, Holland LZ, Laudet V, Schubert M (2006) Retinoic acid signaling and the evolution of chordates. Int J Biol Sci 2:38–47
Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O’Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W (2006) International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58:726–741
Moore DD, Kato S, Xie W, Mangelsdorf DJ, Schmidt DR, Xiao R, Kliewer SA (2006) International Union of Pharmacology. LXII. The NR1H and NR1I receptors: constitutive androstane receptor, pregnene X receptor, farnesoid X receptor α, farnesoid X receptor β, liver X receptor α, liver X receptor β, and vitamin D receptor. Pharmacol Rev 58:742–759
Nuclear Receptors Nomenclature Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell 97:161–163
Paris M, Escriva H, Schubert M, Brunet F, Brtko J, Ciesielski F, Roecklin D, Vivat-Hannah V, Jamin EL, Cravedi JP, Scanlan TS, Renaud JP, Holland ND, Laudet V (2008a) Amphioxus postembryonic development reveals the homology of chordate metamorphosis. Curr Biol 18:825–830
Paris M, Pettersson K, Schubert M, Bertrand S, Pongratz I, Escriva H, Laudet V (2008b) An amphioxus orthologue of the estrogen receptor that does not bind estradiol: insights into estrogen receptor evolution. BMC Evol Biol 8:219
Pereira de Jésus-Tran K, Côté PL, Cantin L, Blanchet J, Labrie F, Breton R (2006) Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci 15:987–999
Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engström O, Ljunggren J, Gustafsson JÅ, Carlquist M (1999) Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J 18:4608–4618
Putnam NH, Butts T, Ferrier DEK, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A, Yu JK, Benito-Gutiérrez È, Dubchak I, Garcia-Fernàndez J, Gibson-Brown JJ, Grigoriev IV, Horton AC, de Jong PJ, Jurka J, Kapitonov V, Kohara Y, Kuroki Y, Lindquist E, Lucas S, Osoegawa K, Pennacchio LA, Salamov AA, Satou Y, Sauka-Spengler T, Schmutz J, Shin- IT, Toyoda A, Bronner-Fraser M, Fujiyama A, Holland LZ, Holland PWH, Satoh N, Rokhsar DS (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453:1064–1072
Schubert M, Escriva H, Xavier-Neto J, Laudet V (2006a) Amphioxus and tunicates as evolutionary model systems. Trends Ecol Evol 21:269–277
Schubert M, Holland ND, Laudet V, Holland LZ (2006b) A retinoic acid-Hox hierarchy controls both anterior/posterior patterning and neuronal specification in the developing central nervous system of the cephalochordate amphioxus. Dev Biol 296:190–202
Sea Urchin Genome Sequencing Consortium (2006) The genome of the sea urchin Strongylocentrotus purpuratus. Science 314:941–952
Tanenbaum DM, Wang Y, Williams SP, Sigler PB (1998) Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc Natl Acad Sci USA 95:5998–6003
Thornton JW, Need E, Crews D (2003) Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science 301:1714–1717
Williams SP, Sigler PB (1998) Atomic structure of progesterone complexed with its receptor. Nature 393:392–396
Yagi K, Satou Y, Mazet F, Shimeld SM, Degnan B, Rokhsar D, Levine M, Kohara Y, Satoh N (2003) A genomewide survey of developmentally relevant genes in Ciona intestinalis. III. Genes for Fox, ETS, nuclear receptors and NFκB. Dev Genes Evol 213:235–244
Yu JK, Satou Y, Holland ND, Shin-I T, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ (2007) Axial patterning in cephalochordates and the evolution of the organizer. Nature 445:613–617
Acknowledgments
The authors would like to thank Michael E. Baker for critical reading of the manuscript. This work was supported by grants from the ANR and the CNRS to M.S., the MENRT to M.S. and V.L. and by CRESCENDO, a European Union Integrated Project of FP6, and by CASCADE, a Network of Excellence of FP6. S.B. is funded by an ARC postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.J. Gibson-Brown
Michael Schubert and Frédéric Brunet are equal first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Sequence IDs and alleles of nuclear hormone receptors (NRs) in the genome of Branchiostoma floridae. (DOC 97.0KB)
Supplementary Table S2
Sequence IDs and alleles of nuclear hormone receptor coregulators (NRCs) in the genome of Branchiostoma floridae.(DOC 41.0KB)
Fig. S1
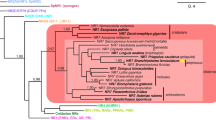
Phylogeny of the nuclear hormone receptor (NR) superfamily. The maximum likelihood tree is based on NRs from humans (Homo sapiens), sea squirts (Ciona intestinalis), fruit flies (Drosophila melanogaster) and amphioxus (Branchiostoma floridae). Human (Homo sapiens) NRs are shown in black, sea squirt (Ciona intestinalis) NRs in green, fruit fly (Drosophila melanogaster) NRs in blue and amphioxus (Branchiostoma floridae) NRs in red. Bootstrap support values are given for each branch (PDF 64.1 KB)
Fig. S2
Phylogeny of the NCOA family. The tree is the result of a maximum likelihood analysis. Bootstrap support values are given for each branch. Sequences are indicated as sequence IDs plus species name. The single amphioxus (Branchiostoma floridae) NCOA homolog is highlighted in red. (PDF 56.0 KB)
Fig. S3
Phylogeny of the CBP and P300 family. The tree is the result of a maximum likelihood analysis. Bootstrap support values are given for each branch. Sequences are indicated as sequence IDs plus species name. The single amphioxus (Branchiostoma floridae) CBP/P300 homolog is highlighted in red (PDF 58.9 KB)
Fig. S4
Phylogeny of the PGC1 family. The tree is the result of a maximum likelihood analysis. Bootstrap support values are given for each branch. Sequences are indicated as sequence IDs plus species name. The single amphioxus (Branchiostoma floridae) PGC1 homolog is highlighted in red (PDF 57.8 KB)
Fig. S5
Phylogeny of the NCOR/SMRT family. The tree is the result of a maximum likelihood analysis. Bootstrap support values are given for each branch. Sequences are indicated as sequence IDs plus species name. The single amphioxus (Branchiostoma floridae) NCOR homolog is highlighted in red (PDF 55.7 KB)
Fig. S6
Phylogeny of the MED1/TRAP220 family. The tree is the result of a maximum likelihood analysis. Bootstrap support values are given for each branch. Sequences are indicated as sequence IDs plus species name. The single amphioxus (Branchiostoma floridae) TRAP220 homolog is highlighted in red (PDF 49.8 KB)
Rights and permissions
About this article
Cite this article
Schubert, M., Brunet, F., Paris, M. et al. Nuclear hormone receptor signaling in amphioxus. Dev Genes Evol 218, 651–665 (2008). https://doi.org/10.1007/s00427-008-0251-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-008-0251-y