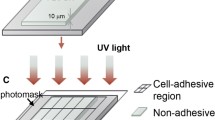
Abstract
Spatially defined growth of cells in culture is a useful model for studies ranging from the characterization of cellular motility to the analysis of network behaviour in structurally defined ensembles of excitable cells. Current methodological approaches for obtaining patterned growth include sophisticated modifications of surface chemistry, stamping techniques and microfluidics. The implementation of most of these techniques requires the availability of highly specialized apparatus and some of the methods are specific for certain cell types and/or substrate materials. The goal of the present study was to develop a cell-patterning technique that can be implemented by any laboratory working with cell culture and that is highly adaptable in terms of cell types and substrate materials. The method is based on a photolithographic process that permits the patterned deposition of attachment factors of choice on surfaces previously coated with agar with a spatial resolution (maximal deviation from a straight line) of ±3 µm. Because agar efficiently prevents cell adhesion, patterned growth obtained with this technique displays virtually no off-pattern cell attachment. The method permitted the patterning of cardiomyocytes, fibroblasts and HeLa cells on either glass substrates or polymer-coated materials with a spatial resolution of a few micrometers.






Similar content being viewed by others
References
Blondel B, Roijen I, Cheneval JP (1971) Heart cells in culture: a simple method for increasing the proportion of myoblasts. Experientia 27:356–358
Branch DW, Corey JM, Weyhenmeyer JA, Brewer GJ, Wheeler BC (1998) Microstamp patterns of biomolecules for high-resolution neuronal networks. Med Biol Eng Comput 36:135–141
Chien CG, Pine J (1991) An apparatus for recording synaptic potentials from neuronal cultures using voltage-sensitive fluorescent dyes. J Neurosci Methods 38:93–105
Chiu DT, Jeon NL, Huang S, Kane RS, Wargo CJ, Choi IS, Ingber DE, Whitesides GM (2000) Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc Natl Acad Sci USA 14:2408–2413
Corey JM, Wheeler BC, Brewer GJ (1996) Micrometer resolution silane-based patterning of hippocampal neurons: critical variables in photoresist and laser ablation processes for substrate fabrication. IEEE Trans Biomed Eng 43:944–955
Detrait E, Lhoest JB, Knoops B, Bertrand P, van den Bosch de Aguilar P (1998) Orientation of cell adhesion and growth on patterned heterogeneous polystyrene surface. J Neurosci Methods 84:193–204
Dostal DE, Booz GW, Baker KM (1996) Angiotensin II signalling pathways in cardiac fibroblasts: conventional versus novel mechanisms in mediating cardiac growth and function. Mol Cell Biochem 157:15–21
Eghbali M (1992) Cardiac fibroblasts: function, regulation of gene expression, and phenotypic modulation. Basic Res Cardiol 87:183–189
Fast VG, Kléber AG (1995) Cardiac tissue geometry as a determinant of unidirectional conduction block: assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc Res 29:697–707
Folch A, Toner M (1998) Cellular micropatterns on biocompatible materials. Biotechnol Prog 14:388–392
Kolega J, Shure MS, Chen WT, Young ND (1982) Rapid cellular translocation is related to close contacts formed between various cultured cells and their substrata. J Cell Sci 54:23–34
Kucera JP, Kléber AG, Rohr S (1998) Slow conduction in cardiac tissue. II. Effects of branching tissue geometry. Circ Res 83:795–805
Kucera JP, Heuschkel MO, Renaud P, Rohr S (2000) Power-law behavior of beat rate variability in monolayer cultures of neonatal rat ventricular myocytes. Circ Res 86:1140–1145
Lieberman M, Horres CR, Shigeto N, Ebihara L, Aiton JF, Johnson EA (1981) Cardiac muscle with controlled geometry. Application to electrophysiological and ion transport studies. In: Nelson PG, Lieberman M (eds) Excitable cells in tissue culture. Plenum, New York, pp 379–408
Rohr S, Salzberg BM (1994) Characterization of impulse propagation at the microscopic level across geometrically defined expansions of excitable tissue. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures. J Gen Physiol 104:287–309
Rohr S, Schölly DM, Kleber AG (1991) Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res 68:114–130
Sheetz MP, Felsenfeld DP, Galbraith CG (1998) Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol 8:51–54
Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE (1994) Engineering cell shape and function. Science 264:696–698
Spach MS, Dolber PC (1986) Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res 58:356–371
Acknowledgements
This study was supported by the Swiss National Science Foundation (Grant Nr. 31-64914.01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohr, S., Flückiger-Labrada, R. & Kucera, J.P. Photolithographically defined deposition of attachment factors as a versatile method for patterning the growth of different cell types in culture. Pflugers Arch - Eur J Physiol 446, 125–132 (2003). https://doi.org/10.1007/s00424-002-1000-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-002-1000-0