Abstract
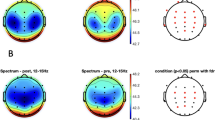
Evidence for a response-control-related kind of declarative memory during deep propofol anesthesia has recently been reported. Connectivity within the mediotemporal lobe (MTL), and in particular rhinal–hippocampal synchronization within the gamma band, has been shown to be crucial for declarative memory formation. Thus, we analyzed EEG recordings obtained from the scalp, as well as directly from within the hippocampus and from the anterior parahippocampal gyrus, which is covered by rhinal cortex, in patients with unilateral temporal lobe epilepsy during propofol anesthesia, which preceded electrode explantation. For the gamma band a power decrease starting with induction of anesthesia was observed at scalp position Cz, but a power increase was detected at MTL locations. In contrast to prior results for sleep recordings, rhinal–hippocampal coherence did not decrease within the gamma band at deeper levels of anesthesia. These findings may represent an indirect electrophysiological correlate of partially intact declarative memory formation during deep propofol sedation. Furthermore, we investigated how well the plasma propofol level, as well as different stages of anesthesia including the burst suppression phase, could be monitored by different spectral as well as by nonlinear EEG measures. We observed that conventional spectral power measures, most prominently those recorded from mediotemporal locations, are most closely correlated with the plasma propofol level, whereas different stages of anesthesia can be distinguished best by nonconventional spectral as well as nonlinear measures.
Similar content being viewed by others
References
Abarbanel HDI, Brown R, Sidorowich JJ, Tsimring LS (1993) The analysis of observed chaotic data in physical systems. Rev Mod Phys 65:1331–1392
Amaral DG, Insausti R (1990) Hippocampal formation. In: Paxinos G (ed) The human nervous system. Academic, San Diego, pp 711–755
Bethune DW, Ghosh S, Gray B, Kerr L, Walker IA, Doolan LA, Harwood RJ, Sharples LD (1992) Learning during general anaesthesia: implicit recall after methohexitone or propofol infusion. Br J Anaesth 69:197–199
Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE (1998) Making memories: Brain activity that predicts how well visual experience will be remembered. Science 281:1185–1187
Bruhn J, Ropcke H, Hoeft A (2000) Approximate entropy as an electroencephalographic measure of anesthetic drug effect during desflurane anesthesia. Anesthesiology 92:715–726
Challis RE, Kitney RI (1991) Biomedical signal processing: Part 3. The power spectrum and coherence function. Med Biol Eng Comput 29:225–241
Cork RC, Heaton JF, Campbell CE, Kihlstrom JF (1996) Is there implicit memory after propofol sedation? Br J Anaesth 76:492–498
Deeprose C, Andrade J, Varma S, Edwards N (2004) Unconscious learning during surgery with propofol anaesthesia. Br J Anaesth 92:171–177
Elger CE, Grunwald T, Lehnertz K, Kutas M, Helmstaedter C, Brockhaus A, Van Roost D, Heinze HJ (1997) Human temporal lobe potentials in verbal learning and memory processes. Neuropsychologia 35:657–667
Engel AK, Singer W (2001) Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 5:16–25
Fell J, Beckmann P (1994) Resonance like phenomena in Lyapunov-calculations from data reconstructed by the time delay method. Phys Lett A 190:172–176
Fell J, Röschke J, Mann K, Schäffner C (1996) Discrimination of sleep stages: a comparison between spectral and nonlinear EEG measures. Electroencephalogr Clin Neurophysiol 98:401–410
Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernández G (2001) Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci 4:1259–1264
Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernández G (2003a) Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma-synchronization? Eur J Neurosci 17:1082–1088
Fell J, Staedtgen M, Burr W, Kockelmann E, Helmstaedter C, Schaller C, Elger CE, Fernández G (2003b) Rhinal-hippocampal EEG coherence is reduced during human sleep. Eur J Neurosci 18:1711–1716
Fernández G, Weyerts H, Schrader-Bölsche M, Tendolkar I, Smid HGOM, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HJ (1998) Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci 18:1841–1847
Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dümpelmann M, van Roost D, Elger CE (1999) Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science 285:1582–1585
Fernández G, Klaver P, Fell J, Grunwald T, Elger CE (2002) Human declarative memory formation: segregating rhinal and hippocampal contributions. Hippocampus 12:514–519
Frank GW, Lookman T, Nerenberg MAH, Essex C, Lemieux J, Blume W (1990) Chaotic time series analyses of epileptic seizures. Physica D 46:427–438
Grassberger P, Schreiber T, Schaffrath C (1991) Nonlinear time sequence analysis. Int J Bifurcat Chaos 1:521–547
Grassberger P, Procaccia I (1983) Measuring the strangeness of strange attractors. Physica D 9:189–208
Grunwald T, Elger CE, Lehnertz K, Van Roost D, Elger CE (1995) Alterations of intrahippocampal cognitive potentials in temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol 95:53–62
Hazeaux C, Tisserant D, Vespignani H, Hummer-Sigiel M, Kwan-Ning V, Laxenaire MC (1987) Electroencephalographic impact of propofol anesthesia. Ann Fr Anesth Reanim 6:261–266
Inouye T, Shinosaki K, Sakamoto H, Toi S, Ukai S, Iyama A, Katsuda Y, Hirano M (1991) Quantification of EEG irregularity by use of the entropy of the power spectrum. Electroencephalogr Clin Neurophysiol 79:204–210
Jacoby LL (1991) A process dissociation framework: separating automatic from intentional uses of memory. J Memory Lang 30:513–541
Keil A, Gruber T, Müller MM (2001) Functional correlates of macroscopic high-frequency brain activity in the human visual system. Neurosci Biobehav Rev 25:527–534
Kerssens C, Lubke CH, Klein J, van der Woerd A, Bonke B (2002) Memory function during propofol and alfentatil anesthesia: predictive value of individual differences. Anesthesiology 97:382–389
Kuizenga K, Kalkman CJ, Hennis PJ (1998) Quantitative electroencephalographic analysis of the biphasic concentration-effect relationship of propofol in surgical patients during extradural analgesia. Br J Anaesth 80:725–732
Marsh B, White M, Morton N, Kenny GN (1991) Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth 67:41–48
Munte S, Kobbe I, Demertzis A, Lullwitz E, Munte TF, Piepenbrock S, Leuwer M (1999) Increased reading speed for stories presented during general anesthesia. Anesthesiology 90:662–669
Paller KA, Mc Carthy G, Roessler E, Allison T, Wood CC (1992) Potentials evoked in human and monkey medial temporal lobe during auditory and visual oddball paradigms. Electroencephalogr Clin Neurophysiol 84:269–279
Puce A, Kalnins RM, Berkovic SF, Donnan GA, Bladin PF (1989) Limbic P3 potentials, seizure localization, and surgical pathology in temporal lobe epilepsy. Ann Neurol 26:377–385
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21
Stapleton CL, Andrade J (2000) An investigation of learning during propofol sedation and anesthesia using the process dissociation procedure. Anesthesiology 93:1418–1425
Steriade M, Amzica F, Contreras D (1994) Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol 90:1–16
Soriano SG, McCann ME, Laussen PC (2002) Neuroanesthesia. Innovative technique and monitoring. Anesthesiol Clin N Am 20:137–151
Takens F (1981) Detecting strange attractors in turbulence. Lect Notes Math 898:366–381
Tallon-Baudry C, Bertrand O (1999) Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3:151–162
Theiler J (1986) Spurious dimensions from correlation algorithms applied to limited time-series data. Phys Rev A 34:2427–2432
Van Gils M, Korhonen I, Yli-Hankala A (2002) Methods for assessing adequacy of anesthesia. Crit Rev Biomed Eng 30:99–130
Van Roost D, Solymosi L, Schramm J, Van Oosterwyck B, Elger CE (1998) Depth electrode implantation in the length axis of the hippocampus for the presurgical evaluation of medial temporal lobe epilepsy: A computed tomography-based stereotactic insertion technique and its accuracy. Neurosurgery 43:819–826
Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001) The brain-web: phase synchronization and large-scale integration. Nat Rev Neurosci 2:229–239
Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL (1998) Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281:1188–1191
Widman G, Schreiber T, Rehberg B, Hoeft A, Elger CE (2000) Quantification of depth of anesthesia by nonlinear time series analysis of brain electrical activity. Phys Rev E 62:4898–4903
Wolf A, Swift JB, Swinney HL, Vastano JA (1985) Determining Lyapunov exponents from a time series. Physica D 16:285–317
Zhang XS, Roy RJ, Jensen EW (2001) EEG complexity as a measure of depth of anesthesia for patients. IEEE Trans Biomed Eng 48:1424–1433
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fell, J., Widman, G., Rehberg, B. et al. Human mediotemporal EEG characteristics during propofol anesthesia. Biol Cybern 92, 92–100 (2005). https://doi.org/10.1007/s00422-004-0538-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-004-0538-7