Abstract
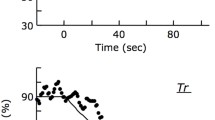
There is some evidence that the fall in intramyocellular oxygen content during ischemic contractions is less than during ischemia alone. We used proton magnetic resonance spectroscopy to determine whether peak deoxy-myoglobin (dMb) obtained during ischemic ankle dorsiflexion contractions attained the maximal dMb level observed during a separate trial of ischemia alone (resting max). In six healthy young men, the rate of myoglobin desaturation was rapid at the onset of ischemic contractions and then slowed as contractions continued, attaining only 75 ± 3.3% (mean ± SE) of resting max dMb by the end of contractions (p = 0.03). Myoglobin continued to desaturate while ischemia was maintained following contractions, reaching 98 ± 1.8% of resting max within 10 min (p = 0.03 vs. end of contractions). Notably, contractions performed after 10 min of ischemia did not affect dMb (dMb = 100 ± 1.5% of resting max, p > 0.99), suggesting that full desaturation had already been achieved. The blunting of desaturation during ischemic contractions is likely a result of slowed mitochondrial oxygen consumption due to limited oxygen availability.




Similar content being viewed by others
References
Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE (2007) Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA 104:1057–1062. doi:10.1073/pnas.0610131104
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their reactions with ligands. In: Neuberger A, Tatum EL (eds) Frontiers of Biology, vol 21. North-Holland, London
Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B (2000) Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol 279:R899–R906
Bergstrom M, Hultman E (1988) Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol 65:1500–1505
Blei ML, Conley KE, Kushmerick MJ (1993) Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465:203–222
Brillault-Salvat C, Giacomini E, Jouvensal L, Wary C, Bloch G, Carlier PG (1997) Simultaneous determination of muscle perfusion and oxygenation by interleaved NMR plethysmography and deoxymyoglobin spectroscopy. NMR Biomed 10:315–323. doi:10.1002/(SICI)1099-1492(199710)10:7<315::AID-NBM489>3.0.CO;2-X
Chasiotis D, Bergstrom M, Hultman E (1987) ATP utilization and force during intermittent and continuous muscle contractions. J Appl Physiol 63:167–174
Connett RJ, Honig CR, Gayeski TE, Brooks GA (1990) Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol 68:833–842
De Blasi RA, Cope M, Ferrari M (1992) Oxygen consumption of human skeletal muscle by near infrared spectroscopy during tourniquet-induced ischemia in maximal voluntary contraction. Adv Exp Med Biol 317:771–777
Duteil S, Bourrilhon C, Raynaud JS, Wary C, Richardson RS, Leroy-Willig A, Jouanin JC, Guezennec CY, Carlier PG (2004) Metabolic and vascular support for the role of myoglobin in humans: a multiparametric NMR study. Am J Physiol Regul Integr Comp Physiol 287:R1441–R1449. doi:10.1152/ajpregu.00242.2004
Gayeski TE, Connett RJ, Honig CR (1987) Minimum intracellular PO2 for maximum cytochrome turnover in red muscle in situ. Am J Physiol 252:H906–H915
Hall N, DeLuca M (1984) The effect of inorganic phosphate on creatine kinase in respiring rat heart mitochondria. Arch Biochem Biophys 229:477–482. doi:10.1016/0003-9861(84)90178-4
Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B (1996) Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol 81:1410–1417
Harkema SJ, Meyer RA (1997) Effect of acidosis on control of respiration in skeletal muscle. Am J Physiol 272:C491–C500
Harris RC, Edwards RH, Hultman E, Nordesjo LO, Nylind B, Sahlin K (1976) The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch 367:137–142. doi:10.1007/BF00585149
Hogan MC, Arthur PG, Bebout DE, Hochachka PW, Wagner PD (1992a) Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol 73:728–736
Hogan MC, Nioka S, Brechue WF, Chance B (1992b) A 31P-NMR study of tissue respiration in working dog muscle during reduced O2 delivery conditions. J Appl Physiol 73:1662–1670
Jubrias SA, Crowther GJ, Shankland EG, Gronka RK, Conley KE (2003) Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol 553:589–599. doi:10.1113/jphysiol.2003.045872
Kreis R, Bruegger K, Skjelsvik C, Zwicky S, Ith M, Jung B, Baumgartner I, Boesch C (2001) Quantitative (1)H magnetic resonance spectroscopy of myoglobin de- and reoxygenation in skeletal muscle: reproducibility and effects of location and disease. Magn Reson Med 46:240–248. doi:10.1002/mrm.1184
Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA (2006) In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577:353–367. doi:10.1113/jphysiol.2006.114249
Mancini DM, Wilson JR, Bolinger L, Li H, Kendrick K, Chance B, Leigh JS (1994) In vivo magnetic resonance spectroscopy measurement of deoxymyoglobin during exercise in patients with heart failure. Demonstration of abnormal muscle metabolism despite adequate oxygenation. Circulation 90:500–508
Mole PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T (1999) Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol 277:R173–R180
Oshino R, Oshino N, Tamura M, Kobilinsky L, Chance B (1972) A sensitive bacterial luminescence probe for O2 in biochemical systems. Biochim Biophys Acta 273:5–17
Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD (1995) Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest 96:1916–1926. doi:10.1172/JCI118237
Richardson RS, Leigh JS, Wagner PD, Noyszewski EA (1999) Cellular PO2 as a determinant of maximal mitochondrial O(2) consumption in trained human skeletal muscle. J Appl Physiol 87:325–331
Richardson RS, Newcomer SC, Noyszewski EA (2001) Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol 91:2679–2685
Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF (1990) Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem 265:15392–15402
Russ DW, Elliott MA, Vandenborne K, Walter GA, Binder-Macleod SA (2002) Metabolic costs of isometric force generation and maintenance of human skeletal muscle. Am J Physiol Endocrinol Metab 282:E448–E457
Schenkman KA, Marble DR, Burns DH, Feigl EO (1997) Myoglobin oxygen dissociation by multiwavelength spectroscopy. J Appl Physiol 82:86–92
Shen J, Rycyna RE, Rothman DL (1997) Improvements on an in vivo automatic shimming method FASTERMAP. Magn Reson Med 38:834–839. doi:10.1002/mrm.1910380521
Sugano T, Oshino N, Chance B (1974) Mitochondrial functions under hypoxic conditions. The steady states of cytochrome c reduction and of energy metabolism. Biochim Biophys Acta 347:340–358. doi:10.1016/0005-2728(74)90074-7
Suleymanlar G, Zhou HZ, McCormack M, Elkins N, Kucera R, Reiss OK, Shapiro JI (1992) Mechanism of impaired energy metabolism during acidosis: role of oxidative metabolism. Am J Physiol 262:H1818–H1822
Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK (1986) Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med 3:44–54. doi:10.1002/mrm.1910030107
Tran TK, Sailasuta N, Kreutzer U, Hurd R, Chung Y, Mole P, Kuno S, Jue T (1999) Comparative analysis of NMR and NIRS measurements of intracellular PO2 in human skeletal muscle. Am J Physiol 276:R1682–R1690
Walsh B, Tiivel T, Tonkonogi M, Sahlin K (2002) Increased concentrations of P(i) and lactic acid reduce creatine-stimulated respiration in muscle fibers. J Appl Physiol 92:2273–2276
Wang ZY, Noyszewski EA, Leigh JS Jr (1990) In vivo MRS measurement of deoxymyoglobin in human forearms. Magn Reson Med 14:562–567. doi:10.1002/mrm.1910140314
Wigmore DM, Damon BM, Pober DM, Kent-Braun JA (2004) MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J Appl Physiol 97:2385–2394. doi:10.1152/japplphysiol.01390.2003
Wilson DF, Owen CS, Erecinska M (1977) Regulation of mitochondrial respiration in intact tissues: a mathematical model. Adv Exp Med Biol 94:279–287
Wilson DF, Erecinska M, Drown C, Silver IA (1979) The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys 195:485–493. doi:10.1016/0003-9861(79)90375-8
Acknowledgments
The authors thank all of the participants, the members of the Muscle Physiology Lab, and Danielle Wigmore PhD, for her assistance with data collection and processing. This study was funded by NIH R01AG21094 and K02AG023582.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tevald, M.A., Lanza, I.R., Befroy, D.E. et al. Intramyocellular oxygenation during ischemic muscle contractions in vivo. Eur J Appl Physiol 106, 333–343 (2009). https://doi.org/10.1007/s00421-009-1021-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1021-x