Abstract
Purpose
To determine the interrelation of different elastic moduli of the cornea and to investigate their dependency on corneal hydration.
Methods
Rabbit eyes were divided into four groups. Corneas were excised and mounted into a Barron artificial anterior chamber. Various corneal hydration steady states were achieved with different dextran T-500 concentrations in the anterior chamber, as well as on the corneal anterior surface. The treatment-solutions of each group contained either 5, 10, 15, or 20% w/w dextran. Ultrasound pachymetry was used to measure central corneal thickness. Brillouin microscopy of the central cornea determined the longitudinal bulk modulus by means of Brillouin frequency shift. Subsequently, a 5-mm-wide central strip was taken for extensiometry to measure the tangential elastic modulus.
Results
The longitudinal bulk modulus was 1.2-times higher in corneas dehydrated with 20% dextran compared to those hydrated with 5% dextran. In contrast, the tangential elastic modulus increased by 4.4 times. The obtained longitudinal bulk moduli were two orders of magnitude bigger than the tangential elastic moduli. Regression analysis of longitudinal bulk modulus and tangential elastic modulus revealed a quadratic relation. The bulk modulus seemed to be independent of tension, whereas the elastic modulus was tension-dependent. Greater corneal hydration led to significantly thicker pachymetry.
Conclusion
Corneal biomechanics are highly dependent on the level of corneal hydration. Surprisingly, tangential elastic moduli were more sensitive to hydration changes than longitudinal bulk moduli. A quadratic relation was found between both moduli.



Similar content being viewed by others
References
Maurice DM (1984) The cornea and the sclera. In: Davson H (ed) The Eye, Vol 1b, 3rd edn. Academic Press, Orlando, pp 1–158
Palko JR, Liu J (2016) Definitions and concepts. In: Roberts CJ, Liu J (eds) Corneal Biomechanics. Kugler Publications, Amsterdam, pp 1–24
Hatami-Marbini H, Maulik R (2016) A biphasic transversely isotropic poroviscoelastic model for the unconfined compression of hydrated soft tissue. J Biomech Eng 138:4032059
Thomsen L (1986) Weak elastic anisotropy. Geophysics 51:1954–1966
Dias J, Ziebarth NM (2015) Impact of hydration media on ex vivo corneal elasticity measurements. Eye Contact Lens 41:281–286
Liu J, Roberts CJ (2005) Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 31:146–155
Spoerl E, Huhle M, Seiler T (1998) Induction of cross-links in corneal tissue. Exp Eye Res 66:97–103
Hoeltzel DA, Altman P, Buzard K, Choe K (1992) Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J Biomech Eng 114:202–215
Hammer A, Richoz O, Mosquera SA, Tabibian D, Hoogewoud F, Hafezi F (2014) Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Invest Ophth Vis Sci 55:2881–2884
Seiler TG, Fischinger I, Senfft T, Schmidinger G, Seiler T (2014) Intrastromal application of riboflavin for corneal crosslinking. Invest Ophthalmol Vis Sci 55:4261–4265
Hatami-Marbini H, Rahimi A (2015) Evaluation of hydration effects on tensile properties of bovine corneas. J Cataract Refract Surg 41:644–651
Boyce BL, Jones RE, Nguyen TD, Grazier JM (2007) Stress-controlled viscoelastic tensile response of bovine cornea. J Biomech 40:2367–2376
Elsheikh A, Alhasso D, Rama P (2008) Biomechanical properties of human and porcine corneas. Exp Eye Res 86:783–790
Elsheikh A, Anderson K (2005) Comparative study of corneal strip extensometry and inflation tests. J R Soc Interface 2:177–185
Gloster J, Perkins ES, Pommier ML (1957) Extensibility of strips of sclera and cornea. Br J Ophthalmol 41:103–110
Mikula ER, Jester JV, Juhasz T (2016) Measurement of an elasticity map in the human cornea. Invest Ophthalmol Vis Sci 57:3282–3286
Scarcelli G, Pineda R, Yun SH (2012) Brillouin optical microscopy for corneal biomechanics. Invest Ophthalmol Vis Sci 53:185–190
Webb JN, Su JP, Scarcelli G (2017) Mechanical outcome of accelerated corneal crosslinking evaluated by Brillouin microscopy. J Cataract Refract Surg 43:1458–1463
Dias JM, Ziebarth NM (2013) Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp Eye Res 115:41–46
Seifert J, Hammer CM, Rheinlaender J, Sel S, Scholz M, Paulsen F, Schäffer TE (2014) Distribution of Young's modulus in porcine corneas after riboflavin/UVA-induced collagen cross-linking as measured by atomic force microscopy. PLoS One 9:e88186
Jue B, Maurice DM (1986) The mechanical properties of the rabbit and human cornea. J Biomech 19:847–853
Boyce BL, Grazier JM, Jones RE, Nguyen TD (2008) Full-field deformation of bovine cornea under constrained inflation conditions. Biomaterials 28:3896–3904
Kling S, Marcos S (2013) Effect of hydration state and storage media on corneal biomechanical response from in vitro inflation tests. J Refract Surg 29:490–497
Hjortdal JO (1996) Regional elastic performance of the human cornea. J Biomech 29:931–942
Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH (2013) Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci 54:1418–1425
Vaughan JM, Randall JT (1980) Brillouin scattering, density and elastic properties of the lens and cornea of the eye. Nature 284:489–491
Soergel F, Jean B, Seiler T, Bende T, Mücke S, Pechhold W, Pels L (1995) Dynamic mechanical spectroscopy of the cornea for measurement of its viscoelastic properties in vitro. Ger J Ophthalmol 4:151–156
Petsche SJ, Chernyak D, Martiz J, Levenston ME, Pinsky PM (2012) Depth-dependent transverse shear properties of the human corneal stroma. Invest Ophthalmol Vis Sci 53:873–880
Spiru B, Kling S, Hafezi F, Sekundo W (2017) Biomechanical differences between femtosecond Lenticule extraction (FLEx) and small incision Lenticule extraction (SmILE) tested by 2D-Extensometry in ex vivo porcine eyes. Invest Ophthalmol Vis Sci 58:2591–2595
Scarcelli G, Yun SH (2016) Brillouin microscopy. In: Roberts CJ, Liu J (eds) Corneal Biomechanics. Kugler Publications, Amsterdam, pp 147–164
Reiß S, Burau G, Stachs O, Guthoff R, Stolz H (2011) Spatially resolved Brillouin spectroscopy to determine the rheological properties of the eye lens. Biomed Opt Express 2:2144–2159
Hedbys BO, Mishima S (1966) The thickness-hydration relationship of the cornea. Exp Eye Res 5:221–228
Hatami-Marbini H, Etebu E (2013) Hydration dependent biomechanical properties of the corneal stroma. Exp Eye Res 116:47–54
Palko JR, Tang J, Cruz Perez B, Pan X, Liu J (2014) Spatially heterogeneous corneal mechanical responses before and after riboflavin-ultraviolet-a crosslinking. J Cataract Refract Surg 40:1021–1031
Müller LJ, Pels E, Schurmans LR, Vrensen GF (2004) A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp Eye Res 78:493–501
Nguyen TD, Jones RE, Boyce BL (2008) A nonlinear anisotropic viscoelastic model for the tensile behavior of the corneal stroma. J Biomech Eng 130:041020
Fratzl P, Daxer A (1993) Structural transformation of collagen fibrils in corneal stroma during drying. An x-ray scattering study. Biophys J 64:1210–1214
Meek KM, Fullwood NJ, Cooke PH, Elliott GF, Maurice DM, Quantock AJ, Wall RS, Worthington CR (1991) Synchrotron x-ray diffraction studies of the cornea, with implications for stromal hydration. Biophys J 60:467–474
Ko MW, Leung LK, Lam DC, Leung CK (2013) Characterization of corneal tangent modulus in vivo. Acta Ophthalmol 91:e263–e269
Singh M, Li J, Han Z, Wu C, Aglyamov SR, Twa MD, Larin KV (2016) Investigating elastic anisotropy of the porcine cornea as a function of intraocular pressure with optical coherence Elastography. J Refract Surg 32:562–567
Sperlich K, Reiß S, Bohn S, Stolz H, Guthoff RF, Jünemann A, Stachs O (2017) Effect of the age-related corneal elasticity on applanation tonometry. Klin Monatsbl Augenheilkd 234:1472–1476
Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE (2006) Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet a light. J Cataract Refract Surg 32:279–283
Elsheikh A, Brown M, Alhasso D, Rama P, Campanelli M, Garway-Heath D (2008) Experimental assessment of corneal anisotropy. J Refract Surg 24:178–187
Acknowledgements
The authors thank Irene E. Kochevar, PhD; Marleen Engler, BSc; and Eric Beck, BSc for their support.
Funding
T. G. Seiler was supported by an unrestricted grant from the Swiss National Science Foundation. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.H. Yun is a co-founder of Intelon Inc., Boston, MA. T. Seiler and P. Shao are scientific consultants of Intelon Inc. T.G. Seiler and B.E. Frueh certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
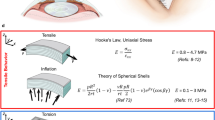
Appendix A: Brillouin frequency shift and elastic module
Appendix A: Brillouin frequency shift and elastic module
Bulk Brillouin scattering can be used to probe elastic properties of transparent materials because incident light is inelastically scattered by acoustic wavelets (phonons) whose velocity is related with the elasticity of the material. Photons of the incident light may take energy from the phonons leading to wavelength shift (Stokes shift) of the scattered light or may deliver energy to the phonons (anti-Stokes shift). Due to the experimental setup, measuring backscattered light perpendicular to the corneal surface, only longitudinal waves can be measured and in this case the Brillouin frequency shift Ω can be expressed by
where n = refractive index of the medium, λ = wavelength, v = velocity of the acoustic wave [31]. Longitudinal ultrasound velocity v in isotropic media, on the other hand, is related with the bulk modulus M
where ρ = density of the medium. This bulk modulus M may not be confused with Young’s elastic modulus E that describes the elastic properties in the surface-parallel plane. Combining Eqs. 1 and 2, it is obvious that M ∝ Ω2, in detail
For a physiologically hydrated cornea (equivalent to 13% dextran) the frequency shift Ω was 5.564 GHz. A density of ρ = 1061 kg/m3, a laser wavelength of λ = 780 nm, and a refractive index of the corneal stroma n = 1.3672 yields for M a value of 2.673 GPa.
Rights and permissions
About this article
Cite this article
Seiler, T.G., Shao, P., Frueh, B.E. et al. The influence of hydration on different mechanical moduli of the cornea. Graefes Arch Clin Exp Ophthalmol 256, 1653–1660 (2018). https://doi.org/10.1007/s00417-018-4069-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4069-7