Abstract
Background
The benzoquinoid antibiotic 17-allylaminogeldanamycin (17-AAG) inhibits the Ras/Raf/MEK and PI3-Kinase signaling pathways and down-regulates vascular endothelial factor expression. Here we use a mouse model of oxygen-induced retinopathy to investigate the effect of 17-AAG on retinal neovascularization and vascular recovery.
Material and methods
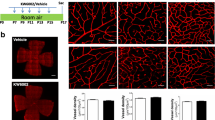
C57BL/6 mice were exposed to 75% oxygen from postnatal day 7 (P7) to P12 and recovered in room air thereafter. Beginning with P12 mice were treated for 5 days by daily IP injection of 17-AAG (12.5 mg/kg body weight) micro dispersed in an emulsion of 4% Lipoid EPC, 5% sucrose, and 0.9% NaCl or Wortmannin (100 μg/kg body weight). On P17, the retinal vascular and avascular area, neovascular blood vessel tufts, and main vessel tortuosity were quantified after perfusion of the mice with FITC-Concanavalin A. The mRNA levels of VEGF, angiopoietin 1 and 2 were quantified by real-time RT-PCR.
Results
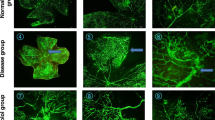
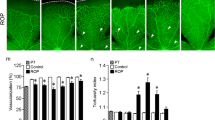
After 17-AAG treatment, a reduction of the vascular area was measured from 37.8±5.2% to 30.8±5.7% (P=0.005), and an increase of the avascular area from 10.8±5.6% to 20.3±6.6% (P=0.001). No alteration of the vascular pattern, the number of blood vessel tufts and the main vessel tortuosity was achieved by treatment with the PI-3 kinase inhibitor Wortmannin. After treatment with 17-AAG, the numbers of tufts (127.9±33.2) were different from the controls (173.7±55.2, P=0.035), but not the main vessel tortuosity. No significant change in VEGF and angiopoietin 1 mRNA expression could be achieved with either of the treatments. Wortmannin treatment also did not change the angiopoietin 2 mRNA level, whereas the level was reduced in 17-AAG treated mice retina from 436-fold (± 64) to 200-fold (±55) (P=0.035).
Conclusion
An IP injection of 17-AAG is able to reduce angioproliferative retinopathy in a mouse model for oxygen-induced retinopathy. Our data indicate that the mechanism does not involve a direct or indirect reduction of the VEGF mRNA level, but acts downstream of the VEGF pathway. Thus, 17-AAG probably does not work by PI-3 kinase inhibition but via the Ras/Raf/MEK pathway. These data underline the potential utility of tyrosine kinase inhibitors in hypoxia induced neovascularization.





Similar content being viewed by others
Abbreviations
- 17-AAG:
-
17-allylaminogeldanamycin
- BW:
-
body weight
- ANG1, ANG2:
-
angiopoietin 1 and 2
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
References
Aiello LP, Northrup JM, Keyt BA, Takagi H (1995) Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 113:1538–1544
Alon T, Hemo I, Itin A, Pe’er J (1995) Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1:1024–1028
Asahara T, Chen D, Takahashi T, Fujikawa K (1998) Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res 83:233–240
Ashton N (1966) Oxygen and the growth and development of retinal vessels. In vivo and in vitro studies. The XX Francis I. Proctor Lecture. Am J Ophthalmol 62:412–435
Basso AD, Solit DB, Munster PN, Rosen N (2002) Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene 21:1159–1166
Brouet A, Sonveaux P, Dessy C, Moniotte S (2001) Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res 89:866–873
Chen JX, Lawrence ML, Cunningham G, Christman BW (2004) HSP90 and Akt modulate Ang-1-induced angiogenesis via NO in coronary artery endothelium. J Appl Physiol 96:612–620
Egorin MJ, Zuhowski EG, Rosen DM, Sentz DL (2001) Plasma pharmacokinetics and tissue distribution of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) in CD2F1 mice1. Cancer Chemother Pharmacol 47:291–302
Fruttiger M (2002) Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci 43:522–527
Glaze ER, Lambert AL, Smith AC, Page JG (2005) Preclinical toxicity of a geldanamycin analog, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), in rats and dogs: potential clinical relevance. Cancer Chemother Pharmacol 56:637–647
Gradin K, McGuire J, Wenger RH, Kvietikova I (1996) Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol 16:5221–5231
Hackett SF, Ozaki H, Strauss RW, Wahlin K (2000) Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol 184:275–284
Hogenesch JB, Chan WK, Jackiw VH, Brown RC (1997) Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem 272:8581–8593
Hur E, Kim HH, Choi SM, Kim JH (2002) Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol 62:975–982
Ishida S, Yamashiro K, Usui T, Kaji Y (2003) Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med 9:781–788
Joussen AM, Poulaki V, Qin W, Kirchhof B (2002) Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 160:501–509
Katschinski DM, Le L, Heinrich D, Wagner KF (2002) Heat induction of the unphosphorylated form of hypoxia-inducible factor-1alpha is dependent on heat shock protein-90 activity. J Biol Chem 277:9262–9267
Kwak YG, Song CH, Yi HK, Hwang PH (2003) Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest 111:1083–1092
Mabjeesh NJ, Post DE, Willard MT, Kaur B (2002) Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res 62:2478–2482
Maisonpierre PC, Suri C, Jones PF, Bartunkova S (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Minet E, Mottet D, Michel G, Roland I (1999) Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett 460:251–256
Murohara T, Asahara T, Silver M, Bauters C (1998) Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101:2567–2578
Nguyen DM, Lorang D, Chen GA, Stewart JH (2001) Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg 72:371–378
Ochel HJ, Eichhorn K, Gademann G (2001) Geldanamycin: the prototype of a class of antitumor drugs targeting the heat shock protein 90 family of molecular chaperones. Cell Stress Chaperones 6:105–112
Penn JS, Tolman BL, Lowery LA (1993) Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci 34:576–585
Pfosser A, Thalgott M, Buttner K, Brouet A (2005) Liposomal Hsp90 cDNA induces neovascularization via nitric oxide in chronic ischemia. Cardiovasc Res 65:728–736
Pichiule P, Chavez JC, LaManna JC (2004) Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J Biol Chem 279:12171–12180
Pierce EA, Avery RL, Foley ED, Aiello LP (1995) Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci 92:905–909
Robert J (2003) Evolution of heat shock protein and immunity. Dev Comp Immunol 27:449–464
Sarlos S, Rizkalla B, Moravski CJ, Cao Z (2003) Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol 163:879–887
Sausville EA, Tomaszewski JE, Ivy P (2003) Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets 3:377–383
Semenza GL (1999) Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15:551–578
Shen BQ, Lee DY, Zioncheck TF (1999) Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. J Biol Chem 274:33057–33063
Silvestre JS, Tamarat R, Ebrahimian TG, Le Roux A (2003) Vascular endothelial growth factor-b promotes in vivo angiogenesis. Circ Res 93:114–123
Simpson DA, Feeney S, Boyle C, Stitt AW (2000) Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative PCR. Mol Vis 6:178–183
Smith LE, Wesolowski E, McLellan A, Kostyk SK (1994) Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35:101–111
Sun J, Liao JK (2004) Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 24:2238–2244
Wenger RH (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16:1151–1162
Zhang H, Burrows F (2004) Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med 82:488–499
Zhang L, Yang N, Park JW, Katsaros D (2003) Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 63:3403–3412
Ziche M, Morbidelli L, Masini E, Amerini S (1994) Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 94:2036–2044
Acknowledgements
The authors thank Claudia Gavranic and Frank Lacina for excellent technical assistance.
Grant information:This study was funded by the Center for Molecular Medicine (CMMC), Cologne; Deutsche Forschungsgemeinschaft DFG Jo 324/6-2; Kämpgen-Stiftung, Cologne; and the Faculty of Medicine of the University of Cologne (Köln Fortune). All authors declare no duality of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kociok, N., Krohne, T.U., Poulaki, V. et al. Geldanamycin treatment reduces neovascularization in a mouse model of retinopathy of prematurity. Graefe's Arch Clin Exp Ophthalmol 245, 258–266 (2007). https://doi.org/10.1007/s00417-006-0355-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-006-0355-x