Abstract
Background
Therapy of autoimmune diseases of the central and peripheral nervous system with intravenous IgG immunoglobulin (IVIg) is well established. Since IVIg is produced from pooled human plasma, autoantibodies can be found in IVIg products and, accordingly, in patient sera after transfusion. The de novo evidence or disappearance of anti-neural autoantibodies after IVIg treatment has so far not been systematically examined.
Methods
We screened 50 neurological patients before and after IVIg treatment for classical onconeural and the most common neurological surface autoantibodies as well as for ganglioside autoantibodies and 23 different antinuclear autoantibodies using immunoblot or cell-based indirect immunofluorescence assays. Furthermore, we screened 31 neurological patients with previously known seropositivity for disappearance of the corresponding antibody after treatment.
Results
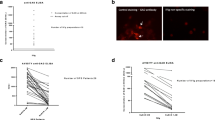
After IVIg treatment, 90% of all sera were de novo positive for antinuclear antibodies, especially for Ro-52. In contrast, 94% of all sera did not show any de novo-positive anti-neural antibodies. In the remaining three cases, titers were very low. Importantly, 12.9% of all tested sera of patients with known antibody positivity turned false negative after IVIg treatment and titers were falsely low in 37% of the remaining sera.
Conclusions
Here, we present for the first time results of a broad screening for clinically relevant autoantibodies before and after IVIg treatment in neurological patients. We identified a high specificity but reduced sensitivity for anti-neural antibody testing after IVIg transfusion. In contrast, antinuclear antibody testing is not reliable after IVIg treatment. These results are of high practical importance for diagnostic of neuroimmunological diseases.
Similar content being viewed by others
References
Chaigne B, Mouthon L (2017) Mechanisms of action of intravenous immunoglobulin. Transfus Apher Sci 56(1):45–49. https://doi.org/10.1016/j.transci.2016.12.017
Arnold DM, Crowther MA, Meyer RM et al (2010) Misleading hepatitis B test results due to intravenous immunoglobulin administration: implications for a clinical trial of rituximab in immune thrombocytopenia. Transfusion 50(12):2577–2581. https://doi.org/10.1111/j.1537-2995.2010.02766.x
Ramsay I, Gorton RL, Patel M et al (2016) Transmission of hepatitis B core antibody and galactomannan enzyme immunoassay positivity via immunoglobulin products: a comprehensive analysis. Clin Infect Dis 63(1):57–63. https://doi.org/10.1093/cid/ciw222
Nixon RR, Smith SA, Johnson RL et al (1994) Misleading hepatitis C serology following administration of intravenous immunoglobulin. Am J Clin Pathol 101(3):327–328
Constable SA, Parry CM, Enevoldson TP et al (2007) Positive serological tests for syphilis and administration of intravenous immunoglobulin. Sex Transm Infect 83(1):57–58. https://doi.org/10.1136/sti.2006.020503
Vora NM, Orciari LA, Bertumen JB et al (2018) Potential confounding of diagnosis of rabies in patients with recent receipt of intravenous immune globulin. MMWR Morb Mortal Wkly Rep 67(5):161–165. https://doi.org/10.15585/mmwr.mm6705a3
Bélanger SS, Fish D, Kim J et al (2012) False-positive human T-lymphotropic virus serology after intravenous immunoglobulin transfusion. CMAJ 184(15):1709–1712. https://doi.org/10.1503/cmaj.120019
Shimizu T, Yarita Y, Suzuki M et al (2005) Serum and urine Helicobacter pylori antibody titer after intravenous gamma-globulin treatment for Kawasaki disease and its clearance. Pediatr Int 47(2):172–174. https://doi.org/10.1111/j.1442-200x.2005.02037.x
Jarius S, Eichhorn P, Albert MH et al (2007) Intravenous immunoglobulins contain naturally occurring antibodies that mimic antineutrophil cytoplasmic antibodies and activate neutrophils in a TNFalpha-dependent and Fc-receptor-independent way. Blood 109(10):4376–4382. https://doi.org/10.1182/blood-2005-12-019604
Jolles S, Deacock S, Turnbull W et al (1999) Atypical C-ANCA following high dose intravenous immunoglobulin. J Clin Pathol 52(3):177–180. https://doi.org/10.1136/jcp.52.3.177
de Beer F, Schreurs MWJ, Foncke EMJ (2009) False positive autoantibodies to glutamic acid decarboxylase in opsoclonus-myoclonus-ataxia syndrome after intravenous treatment with immunoglobulin. Clin Neurol Neurosurg 111(7):643–644. https://doi.org/10.1016/j.clineuro.2009.03.010
Smith TD, Cunningham-Rundles C (2018) Detection of anti-glutamic acid decarboxylase antibodies in immunoglobulin products. J Allergy Clin Immunol Pract 6(1):260–261. https://doi.org/10.1016/j.jaip.2017.04.042
Hao W, Davis C, Daniels T et al (1999) Epitope-specific glutamic acid decarboxylase-65 autoantibodies in intravenous immunoglobulin preparations. Transfus Med 9(4):307–310
van der Molen RG, Hamann D, Jacobs JFM et al (2015) Anti-SSA antibodies are present in immunoglobulin preparations. Transfusion 55(4):832–837. https://doi.org/10.1111/trf.12922
Khan S, Davies L, Cowley D et al (2010) Anti-acetylcholine receptor antibody reactivity of IgG in commercial immunoglobulin preparations. Clin Neurol Neurosurg 112(9):835–836. https://doi.org/10.1016/j.clineuro.2010.06.014
Verstegen G, Duyck MC, Meeus P et al (2009) Detection and identification of antinuclear antibodies (ANA) in a large community hospital. Acta Clin Belg 64(4):317–323. https://doi.org/10.1179/acb.2009.049
Defendenti C, Atzeni F, Spina MF et al (2011) Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimmun Rev 10(3):150–154. https://doi.org/10.1016/j.autrev.2010.09.005
Hayashi N, Koshiba M, Nishimura K et al (2014) Prevalence of disease-specific antinuclear antibodies in general population: estimates from annual physical examinations of residents of a small town over a 5-year period. Mod Rheumatol 18(2):153–160. https://doi.org/10.3109/s10165-008-0028-1
Sali AD, Karakasiliotis I, Evangelidou M et al (2015) Immunological evidence and regulatory potential for cell-penetrating antibodies in intravenous immunoglobulin. Clin Transl Immunol 4(10):e42. https://doi.org/10.1038/cti.2015.18
Ayliffe W, Haeney M, Roberts SC et al (1992) Uveitis after antineutrophil cytoplasmic antibody contamination of immunoglobulin replacement therapy. Lancet 339(8792):558–559
Probst C, Saschenbrecker S, Stoecker W et al (2014) Anti-neuronal autoantibodies: current diagnostic challenges. Mult Scler Relat Disord 3(3):303–320. https://doi.org/10.1016/j.msard.2013.12.001
Lutz HU, Binder CJ, Kaveri S (2009) Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol 30(1):43–51. https://doi.org/10.1016/j.it.2008.10.002
Stathopoulos P, Chastre A, Waters P et al (2019) Autoantibodies against neurologic antigens in nonneurologic autoimmunity. J Immunol 202(8):2210–2219. https://doi.org/10.4049/jimmunol.1801295
Vincent A, Newsom-Davis J (1985) Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: results in 153 validated cases and 2967 diagnostic assays. J Neurol Neurosurg Psychiatry 48(12):1246–1252. https://doi.org/10.1136/jnnp.48.12.1246
Levy Y, Sherer Y, Ahmed A et al (1999) A study of 20 SLE patients with intravenous immunoglobulin-clinical and serologic response. Lupus 8(9):705–712. https://doi.org/10.1191/096120399678841007
Perricone R, de Carolis C, Kröegler B et al (2008) Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology (Oxford) 47(5):646–651. https://doi.org/10.1093/rheumatology/ken046
Monova D, Monov S (2006) Treatment of Active Lupus Nephritis: Intravenous Immunoglobulin G Versus Cyclophosphamide or Azathioprine. BANTAO J 4(2):13
Bain PG, Motomura M, Newsom-Davis J et al (1996) Effects of intravenous immunoglobulin on muscle weakness and calcium-channel autoantibodies in the Lambert–Eaton myasthenic syndrome. Neurology 47(3):678–683. https://doi.org/10.1212/wnl.47.3.678
Dalakas MC (2005) Intravenous immunoglobulin in patients with anti-GAD antibody-associated neurological diseases and patients with inflammatory myopathies: effects on clinicopathological features and immunoregulatory genes. Clin Rev Allergy Immunol 29(3):255–269. https://doi.org/10.1385/CRIAI:29:3:255
Dalakas MC (2006) Role of IVIg in autoimmune, neuroinflammatory and neurodegenerative disorders of the central nervous system: present and future prospects. J Neurol 253(Suppl 5):V25–32. https://doi.org/10.1007/s00415-006-5004-0
Kazatchkine MD, Kaveri SV (2001) Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345(10):747–755. https://doi.org/10.1056/NEJMra993360
Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, Franciotta D, Fujihara K, Jacob A, Kim HJ, Kleiter I, Kumpfel T, Levy M, Palace J, Ruprecht K, Saiz A, Trebst C, Weinshenker BG, Wildemann B (2018) MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 15:134
Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, Franciotta D, Fujihara K, Jacob A, Kim HJ, Kleiter I, Kumpfel T, Levy M, Palace J, Ruprecht K, Saiz A, Trebst C, Weinshenker BG, Wildemann B (2018) MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. Nervenarzt 89:1388–1399
Dressler F, Whalen JA, Reinhardt BN et al (1993) Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 167(2):392–400. https://doi.org/10.1093/infdis/167.2.392
Yeh EA, Nakashima I (2019) Live-cell based assays are the gold standard for anti-MOG-Ab testing. Neurology 92(11):501–502. https://doi.org/10.1212/WNL.0000000000007077
Funding
The study was not funded externally.
Author information
Authors and Affiliations
Contributions
TG participated in serum collection, acquisition of data, analysis and interpretation of data, study design and draft and revision of the manuscript for content. AO and WM performed antibody testing and participated in the revision of the manuscript for content. SJ drafted and revised the manuscript for content. MK participated in serum collection, acquisition of data, analysis and interpretation of data, study design and draft and revision of the manuscript for content. JM participated in serum collection, draft and revision of the manuscript for content. KP and RG drafted and revised the manuscript for content. LK participated in antibody testing, study design and draft and revision of the manuscript for content. IA conceived the idea, designed and directed the project, and participated in serum collection, acquisition of data, analysis and interpretation of data, draft and revision of the manuscript for content.
Corresponding author
Ethics declarations
Conflicts of interest
TG received travel reimbursement from Sanofi Genzyme and Biogen Idec, none related to this manuscript. AO is employee of EUROIMMUN AG, a company that develops, manufactures and markets serological assays for the determination of autoantibodies. WM is employee of EUROIMMUN AG, a company that develops, manufactures and markets serological assays for the determination of autoantibodies. SJ reports no conflicts of interest. MK received a travel grant from Biogen idec, not related to this study. JM received travel grants from Biogen Idec, Novartis AG, Teva and Eisai GmbH, his research is funded by Klaus Tschira Foundation and Ruhr-University, Bochum (FORUM-program); none related to this work. KP received travel funding and speaker honoraria from Biogen Idec, Novartis and Bayer Schering Pharma and funding from the Ruhr-University, Bochum (FORUM-Program), none related to this manuscript. RG serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, and Novartis; has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Bayer Schering Pharma, and Novartis; serves as editor for Therapeutic Advances in Neurological Diseases and on the editorial boards of Experimental Neurology and the Journal of Neuroimmunology; and receives research support from Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, Genzyme, Merck Serono, and Novartis, none related to this manuscript. LK is employee of EUROIMMUN AG, a company that develops, manufactures and markets serological assays for the determination of autoantibodies. IA received travel grants from Biogen Idec and Guthy-Jackson Charitable Foundation, served on scientific advisory boards for Roche and Alexion and received research support from Chugai Pharma, none related to this manuscript.
Ethical standards
The study has been approved by the local ethics committee (Ethik-Kommission Ruhr-Universität Bochum) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Grüter, T., Ott, A., Meyer, W. et al. Effects of IVIg treatment on autoantibody testing in neurological patients: marked reduction in sensitivity but reliable specificity. J Neurol 267, 715–720 (2020). https://doi.org/10.1007/s00415-019-09614-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09614-4