Abstract
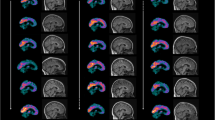
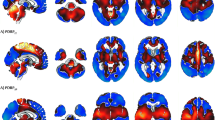
Only one large series using statistical parametric mapping (SPM) reports on FDG-PET in sporadic (Heidenhain and non-Heidenhain variant) Creutzfeldt–Jakob disease (sCJD), describing hypometabolism in bilateral parietal, frontal, and occipital cortices. Our aim was to study FDG-PET in non-Heidenhain probable sCJD patients in order to assess the most pertinent FDG-PET pattern, and to compare FDG-PET and MRI data. We used both SPM and NeuroGam® software analysis, compared with healthy controls, to describe the FDG-PET abnormalities. Individual FDG-PET and MRI–DWI data were compared. SPM group analysis showed lateralized hypometabolism in the medial parietal cortex, the lateral and medial frontal (sparing Brodmann’s area 4 and 6 and the anterior cingulate cortex), and lateral parietal cortex, in the absence of basal ganglia or cerebellar hypometabolism. The most severe hypometabolism was seen in Brodmann’s area 31, and to a lesser degree area 23 (both areas correspond to the posterior cingulate cortex) and the precuneus. On individual analysis using NeuroGam® software, additional variable temporal cortex and frequent basal ganglia (with caudate nucleus as the most frequently involved structure) hypometabolism was seen, in the absence of cerebellar hypometabolism. The cerebral lobe cortex was more frequently and more severely hypometabolic than basal ganglia structures. Concordance between FDG-PET and MRI abnormalities was most often present for both the cerebral lobe cortex and the basal ganglia. In the case of discordance, FDG-PET was more sensitive than MRI for the cortex, whereas MRI was more sensitive than FDG-PET for the basal ganglia. When pathological, both cortical lobe cortex and basal ganglia involvement were slightly more often lateralized on FDG-PET than on MRI. Despite the presence of overlapping features with other diseases presenting with rapidly progressive dementia, the FDG-PET pattern we found in our non-Heidenhain sCJD patients may help in the differential diagnosis of rapidly progressive dementia.


Similar content being viewed by others
References
Zerr I, Kallenberg K, Summers DM et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Brain 132:2659–2668
Na DL, Suh CK, Choi SH et al (1999) Diffusion-weighted magnetic resonance imaging in probable Creutzfeldt–Jakob disease: a clinical-anatomic correlation. Arch Neurol 56:951–957
Engler H, Lundberg PO, Ekbom K et al (2003) Multitracer study with positron emission tomography in Creutzfeldt–Jakob disease. Eur J Nucl Med Mol Imaging 30:85–95
Holthoff VA, Sandmann J, Pawlik G et al (1990) Positron emission tomography in Creutzfeldt–Jakob disease. Arch Neurol 47:1035–1038
Friedland RP, Prusiner SB, Jagust WJ et al (1984) Bitemporal hypometabolism in Creutzfeldt–Jakob disease measured by positron emission tomography with [18F]-2-fluorodeoxyglucose. J Comput Assist Tomogr 8:978–981
Goldman S, Laird A, Flament-Durand J et al (1993) Positron emission tomography and histopathology in Creutzfeldt–Jakob disease. Neurology 43:1828–1830
Matochik JA, Molchan SE, Zametkin AJ et al (1995) Regional cerebral glucose metabolism in autopsy-confirmed Creutzfeldt–Jakob disease. Acta Neurol Scand 91:153–157
Fujita K, Harada M, Sasaki M et al (2012) Multicentre multiobserver study of diffusion-weighted and fluid-attenuated inversion recovery MRI for the diagnosis of sporadic Creutzfeldt–Jakob disease: a reliability and agreement study. BMJ Open 30(2):e000649
Xing XW, Zhang JT, Zhu F et al (2012) Comparison of diffusion-weighted MRI with 18F-fluorodeoxyglucose-positron emission tomography/CT and electroencephalography in sporadic Creutzfeldt–Jakob disease. J Clin Neurosci 19:1354–1357
Kim EJ, Cho SS, Jeong BH et al (2012) Glucose metabolism in sporadic Creutzfeldt–Jakob disease: a statistical parametric mapping analysis of (18) F-FDG PET. Eur J Neurol 19:488–493
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system—an approach to cerebral imaging. Thieme Medical, New York
Paschali A, Messinis L, Lyros E et al (2009) Neuropsychological functions and rCBF SPECT in Parkinson’s disease patients considered candidates for deep brain stimulation. Eur J Nucl Med Mol Imaging 36:1851–1858
Vitali P, Maccagnano E, Caverzasi E et al (2011) Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology 76(20):1711–1719
Cali I, Castellani R, Yuan J et al (2006) Classification of sporadic Creutzfeldt–Jakob disease revisited. Brain 129:2266–2277
Armstrong RA, Cairns NJ, Lantos PL (2001) Quantification of the vacuolation (spongiform change) and prion protein deposition in 11 patients with sporadic Creutzfeldt–Jakob disease. Acta Neuropathol 102:591–596
Berti V, Pupi A, Mosconi L (2011) PET/CT in diagnosis of dementia. Ann N Y Acad Sci 1228:81–92
Herholz K, Salmon E, Perani D et al (2002) Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 17:302–316
Mosconi L, Tsui WH, Herholz K et al (2008) Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med 49:390–398
Dickerson BC, Bakkour A, Salat DH et al (2009) The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 19:497–510
La Joie R, Perrotin A, Barré L et al (2012) Region-specific hierarchy between atrophy, hypometabolism, and ß-amyloid (Aß) load in Alzheimer’s disease dementia. J Neurosci 32:16265–16273
Buckner RL, Sepulcre J, Talukdar T et al (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 29:1860–1873
Mormino EC, Brandel MG, Madison CM et al (2012) Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage 59:1152–1160
Mesulam MM, Van Hoesen GW, Pandya DN et al (1977) Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res 136:393–414
Seltzer B, Pandya DN (1994) Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol 343:445–463
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583
Maquet P (2012) Understanding non rapid eye movement sleep through neuroimaging. World J Biol Psychiatry 11(Suppl 1):9–15
Landolt HP, Glatzel M, Blättler T et al (2006) Sleep-wake disturbances in sporadic Creutzfeldt–Jakob disease. Neurology 9(66):1418–1424
Conflicts of interest
We have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renard, D., Vandenberghe, R., Collombier, L. et al. Glucose metabolism in nine patients with probable sporadic Creutzfeldt–Jakob disease: FDG-PET study using SPM and individual patient analysis. J Neurol 260, 3055–3064 (2013). https://doi.org/10.1007/s00415-013-7117-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7117-6