Abstract
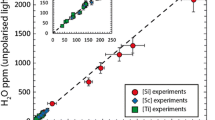
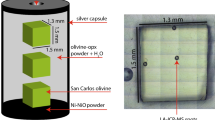
Olivine crystals were grown in the presence of a hydrous silicate fluid during multi-anvil experiments at 8 GPa and 1,000–1,600°C. Experiments were conducted both in a simple system (FeO–MgO–SiO2–H2O) and in a more complex system containing additional elements (CaO–Na2O–Al2O3–Cr2O3–TiO2–FeO–MgO–SiO2–H2O). Silica activity was buffered by the presence of either pyroxene (high a SiO2) or ferropericlase (low a SiO2), and \(f_{{\text{O}}_{\text{2}} }\) was buffered by the presence of Ni + NiO or Fe + FeO, or constrained by the presence of Fe2O3. Raman spectroscopy was used to identify pyroxene polymorphs in the run products. Clinoenstatite was present in the 1,000°C experiment, and enstatite in experiments at 1,400–1,520°C. The H2O content of olivine was measured using secondary ion mass spectroscopy, and infrared spectroscopy was used to investigate the nature of hydrous defects. The H2O storage capacity of olivine decreases with increasing temperature at 8 GPa. In contrast to previous experimental results at ≤2 GPa, no significant effect of varying oxygen fugacity is evident, but H2O storage capacity is enhanced under conditions of low silica activity. No significant growth of low wavenumber (<3,400 cm−1) peaks, generally associated with high \(f_{{\text{O}}_{\text{2}} }\) at low pressure, was observed in the FTIR spectra of olivine from the high \(f_{{\text{O}}_{\text{2}} }\) experiments. Our experiments show that previous high pressure H2O storage capacity measurements for olivine synthesized under more oxidizing conditions than the Earth’s mantle are not likely to be compromised by the \(f_{{\text{O}}_{\text{2}} }\) of the experiments. However, the considerable effect of temperature on H2O storage capacity in olivine must be taken into account to avoid overestimation of the bulk upper mantle H2O storage capacity.




Similar content being viewed by others
References
Aubaud C, Withers AC, Hirschmann MM, Guan YB, Leshin LA, Mackwell SJ, Bell DR (2007) Intercalibration of FTIR and SIMS for hydrogen measurements in glasses and nominally anhydrous minerals. Am Mineral 92:811–828
Bai Q, Kohlstedt DL (1992) Substantial hydrogen solubility in olivine and implications for water storage in the mantle. Nature 357:672–674
Bai Q, Kohlstedt DL (1993) Effects of chemical environment on the solubility and incorporation mechanism for hydrogen in olivine. Phys Chem Mineral 19:460–471
Bell DR, Rossman GR, Maldener J, Endisch D, Rauch F (2003) Hydroxide in olivine: a quantitative determination of the absolute amount and calibration of the IR spectrum. J Geophys Res 108:2105. doi:10.1029/2001JB000679
Bercovici D, Karato S (2003) Whole-mantle convection and the transition-zone water filter. Nature 425:39–44
Berry AJ, Hermann J, O’Neill HStC, Foran GJ (2005) Fingerprinting the water site in mantle olivine. Geology 33:869–872
Berry AJ, O’Neill HStC, Hermann J, Scott DR (2007) The infrared signature of water associated with trivalent cations in olivine. Earth Planet Sci Lett 261:134–142
Bezos A, Humler E (2005) The Fe3+/ΣFe ratios of MORB glasses and their implications for mantle melting. Geochim Cosmochim Acta 69:711–725
Bryndzia LT, Wood BJ (1990) Oxygen thermobarometry of abyssal spinel peridotites—the redox state and C–O–H volatile composition of the earth’s sub-oceanic upper mantle. Am J Sci 290:1093–1116
Chen JH, Inoue T, Yurimoto H, Weidner DJ (2002) Effect of water on olivine-wadsleyite phase boundary in the (Mg, Fe)2SiO4 system. Geophys Res Lett 29:1875. doi:10.1029/2001GL014429
Dasgupta R, Hirschmann MM, Withers AC (2004) Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet Sci Lett 227:73–85
Demouchy S, Deloule E, Frost DJ, Keppler H (2005) Pressure and temperature-dependence of water solubility in Fe-free wadsleyite. Am Mineral 9:1084–1091
Grant KJ, Brooker RA, Kohn SC, Wood BJ (2007) The effect of oxygen fugacity on hydroxyl concentrations and speciation in olivine: implications for water solubility in the upper mantle. Earth Planet Sci Lett 26:217–229
Grant KJ, Kohn SC, Brooker RA (2006) Solubility and partitioning of water in synthetic forsterite and enstatite in the system MgO–SiO2–H2O ± Al2O3. Contrib Mineral Petrol 151:651–664
Hauri EH, Gaetani GA, Green TH (2006) Partitioning of water during melting of the Earth’s upper mantle at H2O-undersaturated conditions. Earth Planet Sci Lett 248:715–734
Hirschmann MM, Aubaud C, Withers AC (2005) Storage capacity of H2O in nominally anhydrous minerals in the upper mantle. Earth Planet Sci Lett 236:167–181
Jarosewich E, Nelen JA, Norberg JA (1980) Reference samples for electron microprobe analysis. Geostandards Newsl 4:43–47
Koga K, Hauri E, Hirschmann M, Bell D (2003) Hydrogen concentration analyses using SIMS and FTIR: comparison and calibration for nominally anhydrous minerals. Geochem Geophy Geosy 4:1019. doi:10.1029/2002GC000378
Kohlstedt DL, Keppler H, Rubie DC (1996) Solubility of water in the alpha, beta and gamma phases of (Mg, Fe) 2SiO4. Contrib Mineral Petrol 123:345–357
Lemaire C, Kohn SC, Brooker RA (2004) The effect of silica activity on the incorporation mechanisms of water in synthetic forsterite: a polarised infrared spectroscopic study. Contrib Mineral Petrol 147:48–57
Libowitzky E, Rossman GR (1996) Principles of quantitative absorbance measurements in anisotropic crystals. Phys Chem Mineral 23:319–327
Litasov KD, Ohtani E, Kagi H, Jacobsen SD, Ghosh S (2007) Temperature dependence and mechanism of hydrogen incorporation in olivine at 12.5–14.0 GPa. Geophys Res Lett 34:L16314. doi:10.1029/2007GL030737
Locke DR, Holloway JR, Leinenweber K, Hervig R (2001) Effect of MgO/SiO2 activity ratio on H2O solubility in forsterite. EOS Transactions AGU, 82 (47), Abstract T21B-0890
Matveev S, O’Neill HS, Ballhaus C, Taylor WR, Green DH (2001) Effect of silica activity on OH-IR spectra of olivine: implications for low-aSiO2 mantle metasomatism. J Petrol 42:721–729
McCammon C (2005) The paradox of mantle redox. Science 308:807–808
Mosenfelder JL, Deligne NI, Asimow PD, Rossman GR (2006) Hydrogen incorporation in olivine from 2–12 GPa. Am Mineral 91:285–294
Paterson MS (1982) The determination of hydroxyl by infrared-absorption in quartz, silicate-glasses and similar materials. Bull Mineral 105:20–29
Smyth JR, Frost DJ, Nestola F, Holl CM, Bromiley G (2006) Olivine hydration in the deep upper mantle: effects of temperature and silica activity. Geophys Res Lett 33:L15301. doi:10.11029/12006GL026194
Stalder R, Ulmer P, Thompson AB, Gunther D (2001) High pressure fluids in the system MgO–SiO2–H2O under upper mantle conditions. Contrib Mineral Petrol 140:607–618
Stixrude L, Lithgow-Bertelloni C (2005) Mineralogy and elasticity of the oceanic upper mantle: origin of the low-velocity zone. J Geophys Res 110:B03204
Ulmer P, Stalder R (2001) The Mg(Fe) SiO3 orthoenstatite-clinoenstatite transitions at high pressures and temperatures determined by Raman spectroscopy on quenched samples. Am Mineral 86:1267–1274
Williams Q, Hemley RJ (2001) Hydrogen in the deep earth. Ann Rev Earth Planet Sci 29:365–418
Withers AC, Hirschmann MM (2007) H2O storage capacity of MgSiO3 clinoenstatite at 8–13 GPa, 1,100–1,400°C. Contrib Mineral Petrol 15:663–674
Zhao YH, Ginsberg SB, Kohstedt DL (2004) Solubility of hydrogen in olivine: dependence on temperature and iron content. Contrib Mineral Petrol 14:155–161
Acknowledgments
We thank David Bell and an anonymous reviewer for their careful reading and appraisal of this paper. We are indebted to C.L. Dodgson for reassuring us that our use of terminology relating to storage capacity is appropriate. Parts of this work were carried out in the University of Minnesota I.T. Characterization Facility, which receives partial support from NSF through the NNIN program. This work was supported by NSF EAR0456405 and OCE 0623550.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T.L. Grove.
Rights and permissions
About this article
Cite this article
Withers, A.C., Hirschmann, M.M. Influence of temperature, composition, silica activity and oxygen fugacity on the H2O storage capacity of olivine at 8 GPa. Contrib Mineral Petrol 156, 595–605 (2008). https://doi.org/10.1007/s00410-008-0303-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-008-0303-3