Abstract
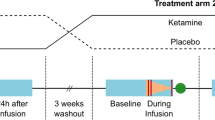
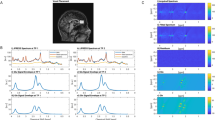
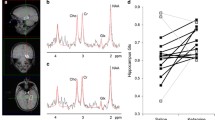
Ketamine exerts rapid antidepressant effects peaking 24 h after a single infusion, which have been suggested to be reflected by both reduced functional connectivity (FC) within default mode network (DMN) and altered glutamatergic levels in the perigenual anterior cingulate cortex (pgACC) at 24 h. Understanding the interrelation and time point specificity of ketamine-induced changes of brain circuitry and metabolism is thus key to future therapeutic developments. We investigated the correlation of late glutamatergic changes with FC changes seeded from the posterior cingulate cortex (PCC) and tested the prediction of the latter by acute fractional amplitude of low-frequency fluctuations (fALFF). In a double-blind, randomized, placebo-controlled study of 61 healthy subjects, we compared effects of subanesthetic ketamine infusion (0.5 mg/kg over 40 min) on resting-state fMRI and MR-Spectroscopy at 7 T 1 h and 24 h post-infusion. FC decrease between PCC and dorsomedial prefrontal cortex (dmPFC) was found at 24 h post-infusion (but not 1 h) and this FC decrease correlated with glutamatergic changes at 24 h in pgACC. Acute increase in fALFF was found in ventral PCC at 1 h which was not observed at 24 h and inversely correlated with the reduced dPCC FC towards the dmPFC at 24 h. The correlation of metabolic and functional markers of delayed ketamine effects and their temporal specificity suggest a potential mechanistic relationship between glutamatergic modulation and reconfiguration of brain regions belonging to the DMN.


Similar content being viewed by others
References
Berman RM, Cappiello A, Anand A et al (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. https://doi.org/10.1016/S0006-3223(99)00230-9
Murrough JW, Iosifescu DV, Chang LC et al (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. https://doi.org/10.1176/appi.ajp.2013.13030392
Zarate CA, Singh JB, Carlson PJ et al (2006) A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864
Berman MG, Peltier S, Nee DE et al (2011) Depression, rumination and the default network. Soc Cogn Affect Neurosci 6:548–555. https://doi.org/10.1093/scan/nsq080
Greicius MD, Flores BH, Menon V et al (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437. https://doi.org/10.1016/j.biopsych.2006.09.020
Sheline YI, Price JL, Yan Z, Mintun MA (2010) Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci 107:11020–11025. https://doi.org/10.1073/pnas.1000446107
Sheline YI, Barch DM, Price JL et al (2009) The default mode network and self-referential processes in depression. Proc Natl Acad Sci 106:1942–1947
Perrin JS, Merz S, Bennett DM et al (2012) Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci 109:5464–5468. https://doi.org/10.1073/pnas.1117206109
Lv Q, Yang L, Li G et al (2015) Large-scale persistent network reconfiguration induced by ketamine in anesthetized monkeys: relevance to mood disorders. Biol Psychiatry 179:765–775. https://doi.org/10.1016/j.biopsych.2015.02.028
Scheidegger M, Walter M, Lehmann M et al (2012) Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7:e44799. https://doi.org/10.1371/journal.pone.0044799
Walter M, Li S, Demenescu LR (2014) Multistage drug effects of ketamine in the treatment of major depression. Eur Arch Psychiatry Clin Neurosci 264:55–65. https://doi.org/10.1007/s00406-014-0535-3
Luykx JJ, Laban KG, van den Heuvel MP et al (2012) Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of 1H-MRS findings. Neurosci Biobehav Rev 36:198–205. https://doi.org/10.1016/j.neubiorev.2011.05.014
Li M, Demenescu LR, Colic L et al (2016) Temporal dynamics of antidepressant ketamine effects on glutamine cycling follow regional fingerprints of AMPA and NMDA receptor densities. Neuropsychopharmacology 42:npp2016184. https://doi.org/10.1038/npp.2016.184
Yüksel C, Öngür D (2010) Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 68:785–794. https://doi.org/10.1016/j.biopsych.2010.06.016
Zhao J, Verwer RWH, van Wamelen DJ et al (2016) Prefrontal changes in the glutamate-glutamine cycle and neuronal/glial glutamate transporters in depression with and without suicide. J Psychiatr Res 82:8–15. https://doi.org/10.1016/j.jpsychires.2016.06.017
Milak MS, Proper CJ, Mulhern ST et al (2016) A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry 21:320–327. https://doi.org/10.1038/mp.2015.83
Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ (2012) Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol (Oxf) 26:733–737. https://doi.org/10.1177/0269881111405359
Zarate CA Jr, Brutsche N, Laje G et al (2012) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338. https://doi.org/10.1016/j.biopsych.2012.03.004
Zhao X, Venkata SLV, Moaddel R et al Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol 74:304–314. https://doi.org/10.1111/j.1365-2125.2012.04198.x
Maeng S, Zarate CA, Du J et al (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. https://doi.org/10.1016/j.biopsych.2007.05.028
Miller OH, Yang L, Wang C-C et al (2014) GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife 3:e03581. https://doi.org/10.7554/eLife.03581
Zanos P, Moaddel R, Morris PJ et al (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. https://doi.org/10.1038/nature17998
Duman RS (2014) Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci 16:11–27
Palomero-Gallagher N, Vogt BA, Schleicher A et al (2009) Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp 30:2336–2355. https://doi.org/10.1002/hbm.20667
Walter M, Henning A, Grimm S et al (2009) The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66:478–486
Horn DI, Yu C, Steiner J et al (2010) Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 4:1–10. https://doi.org/10.3389/fnsys.2010.00033
Li M, Demenescu LR, Colic L et al (2017) Temporal dynamics of antidepressant ketamine effects on glutamine cycling follow regional fingerprints of AMPA and NMDA receptor densities. Neuropsychopharmacology 42:1201–1209. https://doi.org/10.1038/npp.2016.184
Deakin JW, Lees J, McKie S et al (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco–magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164
Långsjö JW, Salmi E, Kaisti KK et al (2004) Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology 100:1065–1071
Vollenweider FX, Leenders KL, Scharfetter C et al (1997) Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F] fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 7:9–24
Aiello M, Salvatore E, Cachia A et al (2015) Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. NeuroImage 113:111–121. https://doi.org/10.1016/j.neuroimage.2015.03.017
Nugent AC, Martinez A, D’Alfonso A et al (2015) The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J Cereb Blood Flow Metab 35:583–591
Zou Q-H, Zhu C-Z, Yang Y et al (2008) An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172:137–141. https://doi.org/10.1016/j.jneumeth.2008.04.012
Esterlis I, DellaGioia N, Pietrzak RH et al (2017) Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C]ABP688 and PET imaging study in depression. Mol Psychiatry. https://doi.org/10.1038/mp.2017.58
Chung J-Y, In M-H, Oh S-H et al (2011) An improved PSF mapping method for EPI distortion correction in human brain at ultra high field (7 T). Magn Reson Mater Phys Biol Med 24:179–190. https://doi.org/10.1007/s10334-011-0251-1
In M-H, Speck O (2012) Highly accelerated PSF-mapping for EPI distortion correction with improved fidelity. Magn Reson Mater Phys Biol Med 25:183–192. https://doi.org/10.1007/s10334-011-0275-6
Power JD, Barnes KA, Snyder AZ et al (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018
Leech R, Sharp DJ (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. https://doi.org/10.1093/brain/awt162
Dou W, Speck O, Benner T et al (2015) Automatic voxel positioning for MRS at 7 T. Magn Reson Mater Phys Biol Med 28:259–270. https://doi.org/10.1007/s10334-014-0469-9
Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1602413113
Abdallah CG, Averill LA, Collins KA et al (2017) Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. https://doi.org/10.1038/npp.2016.186
Kraguljac NV, Frölich MA, Tran S et al (2017) Ketamine modulates hippocampal neurochemistry and functional connectivity—a combined magnetic resonance spectroscopy and resting state fMRI study in healthy volunteers. Mol Psychiatry 22:562–569. https://doi.org/10.1038/mp.2016.122
Salvadore G, Zarate CA (2010) Magnetic resonance spectroscopy studies of the glutamatergic system in mood disorders: a pathway to diagnosis. Novel therapeutics personalized medicine? Biol Psychiatry 68:780–782. https://doi.org/10.1016/j.biopsych.2010.09.011
Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. https://doi.org/10.1126/science.1222939
Vogt BA, Vogt L, Laureys S (2006) Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage 29:452–466. https://doi.org/10.1016/j.neuroimage.2005.07.048
Braga RM, Buckner RL (2017) Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron 95:457–471.e5. https://doi.org/10.1016/j.neuron.2017.06.038
Fox MD, Snyder AZ, Vincent JL et al (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678
Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651
Nugent AC, Ballard ED, Gould TD et al (2018) Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. https://doi.org/10.1038/s41380-018-0028-2
Evans JW, Szczepanik J, Brutsché N et al (2018) Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2018.01.027
Stone J, Dietrich C, Edden R et al (2012) Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 17:664–665. https://doi.org/10.1038/mp.2011.171
Glue P, Gulati A, Nedelec ML, Duffull S (2011) Dose- and exposure-response to ketamine in depression. Biol Psychiatry 70:e9–e10. https://doi.org/10.1016/j.biopsych.2010.11.031
Larkin GL, Beautrais AL (2017) A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol 20:611–611. https://doi.org/10.1093/ijnp/pyx035
Lai R, Katalinic N, Glue P et al (2014) Pilot dose–response trial of i.v. ketamine in treatment-resistant depression. World J Biol Psychiatry 15:579–584. https://doi.org/10.3109/15622975.2014.922697
Loo CK, Gálvez V, O’Keefe E et al (2016) Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 134:48–56. https://doi.org/10.1111/acps.12572
Shehzad Z, Kelly AMC, Reiss PT et al (2009) The resting brain: unconstrained yet reliable. Cereb Cortex 19:2209–2229. https://doi.org/10.1093/cercor/bhn256
Cole DM, Smith SM, Beckmann CF (2010) Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. https://doi.org/10.3389/fnsys.2010.00008
Acknowledgements
This work was supported by German Research Foundation (SFB 779/A06 for AF and MWa, and DFG Wa 2673/4-1 for MWa), the Centre for Behavioural and Brain Sciences (CBBS NN05 for AF and MWa), and Leibniz Association (Pakt für Forschung und Innovation for AF and MWa). MWo was supported by a scholarship from the Medical Faculty of the Otto-von-Guericke-University Magdeburg and German Academic Exchange Service (DAAD). LC received a scholarship from German Research Foundation (SFB 779, 2013–2016). We would like to thank Dr. Claus Tempelmann and Renate Blobel (Department of Neurology) for data acquisition; Dr. Melanie Weigel (Department of Ophthalmology) and Dr. Conrad Friedrich Genz (Department of Cardiology) for clinical screening; Dr. Julia Noack and Linda Frolik Endrulat (Clinical Affective Neuroimaging Laboratory) for trial management. We would like to acknowledge and thank all participants in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MWa received research support from HEEL and Janssen Pharmaceutical Research for a clinical trial on ketamine in patients with major depression which were not investigated in this manuscript. GS is employed at Janssen Research and Development. OS has received research support from Siemens Healthcare, which is not related to this study. Other authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, M., Woelfer, M., Colic, L. et al. Default mode network connectivity change corresponds to ketamine’s delayed glutamatergic effects. Eur Arch Psychiatry Clin Neurosci 270, 207–216 (2020). https://doi.org/10.1007/s00406-018-0942-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0942-y