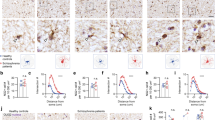
Abstract
Profound white matter abnormalities have repeatedly been described in schizophrenia, which involve the altered expression of numerous oligodendrocyte-associated genes. Transcripts of the disrupted-in-schizophrenia 1 (DISC1) gene, a key susceptibility factor in schizophrenia, have recently been shown to be expressed by oligodendroglial cells and to negatively regulate oligodendrocyte differentiation and maturation. To learn more about the putative role(s) of oligodendroglia-associated DISC1 in schizophrenia, we analyzed the density of DISC1-immunoreactive oligodendrocytes in the fronto-parietal white matter in postmortem brains of patients with schizophrenia. Compared with controls (N = 12) and cases with undifferentiated/residual schizophrenia (N = 6), there was a significantly increased density of DISC1-expressing glial cells in paranoid schizophrenia (N = 12), which unlikely resulted from neuroleptic treatment. Pathophysiologically, over-expression of DISC1 protein(s) in white matter oligodendrocytes might add to the reduced levels of two myelin markers, 2′,3′-cyclic-nucleotide 3′-phosphodiesterase and myelin basic protein in schizophrenia. Moreover, it might significantly contribute to cell cycle abnormalities as well as to deficits in oligodendroglial cell differentiation and maturation found in schizophrenia.




Similar content being viewed by others
Abbreviations
- CPZ:
-
Chlorpromazine
- DAB:
-
3, 3′-diaminobenzidine
- DISC1 :
-
Disrupted-in-schizophrenia 1
- OLs:
-
Oligodendrocytes
- n.a:
-
Not available
- PBS:
-
Phosphate-buffered saline
References
Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B (2015) Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res 161:4–18
Griffa A, Baumann PS, Ferrari C, Do KQ, Conus P, Thiran JP, Hagmann P (2015) Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum Brain Mapp 36:354–366
Najjar S, Pearlman DM (2015) Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 16:102–112
Parlapani E, Schmit A, Erdmann A, Bernstein HG, Breunig B, Gruber O, Petroianu G, von Wilmsdorff M, Schneider-Axmann T, Honer W, Falkai P (2009) Association between myelin basic protein expression and left entorhinal cortex pre-alpha cell layer disorganization in schizophrenia. Brain Res 1301:126–134
Schmitt A, Hasan A, Gruber O, Falkai P (2011) Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci Suppl 2:S150–S154
Walterfang M, Velakoulis D, Whitford TJ, Pantelis C (2011) Understanding aberrant white matter development in schizophrenia: an avenue for therapy? Expert Rev Neurother 11:971–987
Mighdoll MI, Tao R, Kleinman JE, Hyde TM (2015) Myelin, myelin-related disorders, and psychosis. Schizophr Res 161:85–93
Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC (2006) DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol 497:436–450
Bertram I, Bernstein HG, Lendeckel U, Bukowska A, Dobrowolny H, Keilhoff G, Kanakis D, Mawrin C, Bielau H, Falkai P, Bogerts B (2007) Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefronta cortex in schizophrenia and in unipolar depression. Ann NY Acad Sci 1096:147–156
Kostović I, Judaš M, Sedmak G (2011) Developmental history of the subplate zone, subplate neurons and interstitial white matter neurons: relevance for schizophrenia. Int J Dev Neurosci 29:193–205
Yang Y, Fung SJ, Rothwell A, Tianmei S, Weickert CS (2011) Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biol Psychiatry 69:63–70
Byne W, Kidkardnee S, Tatusov SA, Yiannoulos G, Buchsbaum MS, Haroutunian V (2006) Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 85:245–253
Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD (2002) Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res 27:1193–1200
Hof PR, Haroutunian V, Friedrich VL Jr, Byne W, Buitron C, Perl DP, Davis KL (2003) Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 53:1075–1085
Uranova NA, Orlovskaya DD, Vikhreva OV, Zimina IS, Rakhmanova VI (2001) Morphometric study of ultrastructural changes in oligodendroglial cells in the postmortem brain in endogenous psychoses. Vestn Ross Akad Med Nauk 7:42–48
Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova V (2004) Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 67:269–275
Bernstein HG, Steiner J, Bogerts B (2009) Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert Rev Neurother 9:1059–1071
Bernstein HG, Smalla KH, Dürrschmidt D, Keilhoff G, Dobrowolny H, Steiner J, Schmitt A, Kreutz MR, Bogerts B (2012) Increased density of prohibitin-immunoreactive oligodendrocytes in the dorsolateral prefrontal white matter of subjects with schizophrenia suggests extraneuronal roles for the protein in the disease. Neuromolecular Med 14:270–280
Höistad M, Sega D, Takahashi N, Sakurai T, Buxbaum JD, Hof PR (2009) Linking white and grey matter in schizophrenia: oligodendrcyte and neuron pathology in the prefrontal cortex. Front Neuroanat 3:9
Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WIF, Bogerts B, Schmitz C, Falkai P (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117:395–407
Farkas N, Lendeckel U, Dobrowolny H, Funke S, Steiner J, Keilhoff G, Schmitt A, Bogerts B, Bernstein HG (2010) Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry 11:556–566
Vostrikov V, Uranova N (2011) Age-related increase in the number of oligodendrocytes is dysregulated in schizophrenia and mood disorders. Schizophr Res Treat 2011:174689
Miyata S, Hattori T, Shimizu S, Ito A, Tohyama M (2015) Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. Biomed Res Int 2015:492367
Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ (2000) Disruption of two novel genes by a transloation co-segregating with schizophrenia. Mol Genet 9:1415–1423
Thomson PA, Parla JS, McRae AF, Kramer M, Ramakrishnan K, Yao J, Soares DC, McCarthy S, Morris SW, Cardone L, Cass S, Ghiban E, Hennah W, Evans KL, Rebolini D, Millar JK, Harris SE, Starr JM, MacIntyre DJ, Generation Scotland, McIntosh AM, Watson JD, Deary IJ, Visscher PM, Blackwood DH, McCombie WR, Porteous DJ (2014) 708 common and 2010 rare DISC1 locus variants identified in 1542 subjects: analysis for association with psychiatric disorder and cognitive traits. Mol Psychiatry 19:668–675
Porteous DJ, Millar JK (2006) Disrupted in schizophrenia 1: building brains and memories. Trends Mol Med 12:255–261
Lee SA, Kim SM, Suh BK, Sun HY, Park YU, Hong JH, Park C, Nguyen MD, Nagata K, Yoo JY, Park SK (2015) Disrupted-in-schizophrenia 1 (DISC1) regulates dysbindin function by enhancing its stability. J Biol Chem 290:7087–7096
Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Konneker T, Lin W, Styner M, Gilmore JH (2014) Common variants in psychiatric risk genes predict brain structure at birth. Cereb Cortex 24:1230–1246
Brauns S, Gollub RL, Roffman JL, Yendik A, Ho BC, Wassink TH, Heinz A, Ehrlich S (2011) DISC1 is associated with cortical thickness and neural efficiency. Neuroimage 57:1591–1600
Kirkpatrick B, Messias NC, Conley RR, Roberts RC (2003) Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficient and nondeficient schizophrenia. J Nerv Ment Dis 191:563–567
Seshadri S, Kamiya A, Yokota Y, Prikulis I, Kano S, Hayashi-Takagi A, Stanco A, Eom TY, Rao S, Ishizuka K, Wong P, Korth C, Anton ES, Sawa A (2010) Disrupted-in-Schizophrenia-1 expression is regulated by beta-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade. Proc Natl Acad Sci USA 107:5622–5627
Abazyan S, Yang EJ, Abazyan B, Xia M, Yang C, Rojas C, Slusher B, Sattler R, Pletnikov M (2014) Mutant disrupted-in-schizophrenia 1 in astrocytes: focus on glutamate metabolism. J Neurosci Res 92:1659–1668
Yamamuro K, Kimoto S, Rosen KM, Kishimoto T, Makinodan M (2015) Potential primary roles of glial cells in the mechanisms of psychiatric disorders. Front Cell Neurosci 9:154. doi:10.3389/fncel.2015.00154.eCollection
Katsel P, Tan W, Abazyan B, Davis KL, Ross C, Pletnikov MV, Haroutunian V (2011) Expression of mutant human DISC1 in mice supports abnormalities in differentiation of oligodendrocytes. Schizophr Res 130:238–249
Hattori T, Shimizu S, Koyama Y, Emoto H, Matsumoto Y, Kumamoto N, Yamada K, Takamura H, Matsuzaki S, Katayama T, Tohyama M, Ito A (2014) DISC1 (disrupted-in-schizophrenia-1) regulates differentiation of oligodendrocytes. PLoS One 9:e88506
Sprooten E, Sussmann JE, Moorhead TW, Whalley HC, Ffrench-Constant C, Blumberg HP, Bastin ME, Hall J, Lawrie SM, McIntosh AM (2011) Association of white matter integrity with genetic variation in an exonic DISC1 SNP. Mol Psychiatry 16(685):688–689
Duff BJ, Macritchie KA, Moorhead TW, Lawrie SM, Blackwood DH (2013) Human brain imaging studies of DISC1 in schizophrenia, bipolar disorder and depression: a systematic review. Schizophr Res 47:1–13
Whalley HC, Dimitrova R, Sprooten E, Dauvermann MR, Romaniuk L, Duff B, Watson AR, Moorhead B, Bastin M, Semple SI, Giles S, Hall J, Thomson P, Roberts N, Hughes ZA, Brandon NJ, Dunlop J, Whitcher B, Blackwood DH, McIntosh AM, Lawrie SM (2015) Effects of a balanced translocation between chromosomes 1 and 11 disrupting the DISC1 locus on white matter integrity. PLoS One 10:e0130900 (eCollection 2015)
Burns J, Job D, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM (2003) Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry 182:439–443
Spalletta G, Tomaiuolo F, Marino V, Bonaviri G, Trequattrini A, Caltagirone C (2003) Chronic schizophrenia as a brain misconnection syndrome: a white matter voxel-based morphometry study. Schizophr Res 64:15–23
Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L (2005) Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage 27:753–770
Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Hazlett EA, Haznedar MM, Buchsbaum MS (2007) A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. Neuroimage 37:449–462
Kyriakopoulos M, Frangou S (2009) Recent diffusion tensor imaging findings in early stages of schizophrenia. Curr Opin Psychiatry 22:168–176
Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B (1999) Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci 11:71–78
Bernstein HG, Stricker R, Lendeckel U, Bertram I, Dobrowolny H, Steiner J, Bogerts B, Reiser G (2009) Reduced neuronal co-localisation of nardilysin and the putative alpha-secretases ADAM10 and ADAM17 in Alzheimer’s disease and Down syndrome brains. Age (Dordr) 31:11–25
Bernstein HG, Baumann B, Danos P, Diekmann S, Bogerts B, Gundelfinger ED, Braunewell KH (1999) Regional and cellular distribution of neural visinin-like protein immunoreactivities (VILIP-1 and VILIP-3) in human brain. J Neurocytol 28:655–662
Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B (1998) Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience 83:867–875
Vázquez-Bourgon J, Mata I, Roiz-Santiáñez R, Ayesa-Arriola R, Suárez Pinilla P, Tordesillas-Gutiérrez D, Vázquez-Barquero JL, Crespo-Facorro B (2014) Disrupted-in-Schizophrenia 1 gene variant is associated with clinical symptomatology in patients with first-episode psychosis. Psychiatry Investig 11:186–191
Szeszko PR, Hodgkinson CA, Robinson DG, Derosse P, Bilde RM, Lencz T, Burdick KE, Napolitano B, Betensky JD, Kane JM, Goldman D, Malhotra AK (2008) DISC1 is associated with prefrontal cortical gray matter and positive symptoms in schizophrenia. Biol Psychol 79:103–110
Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, Smit AB, Posthuma D, Verheijen MH (2014) Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull 40:925–935
Martins-de-Souza D (2010) Proteome and transcriptome analysis suggests oligodendrocyte dysfunction in schizophrenia. J Psychiatr Res 44:149–156
Le Hellard S, Mühleisen TW, Djurovic S, Fernø J, Ouriaghi Z, Mattheisen M, Vasilescu C, Raeder MB, Hansen T, Strohmaier J, Georg A, Brockschmidt FF, Melle I, Nenadic I, Sauer H, Rietschel M, Nöthen MM, Werge T, Andreassen OA, Cichon S, Steen VM (2010) Polymorphisms in SREBF1 and SREBF2, two antipsychotic-activated transcription factors controlling cellular lipogenesis, are associated with schizophrenia in German and Scandinavian samples. Mol Psychiatry 15:463–472
Hakak Y, Walker JR, Li C, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA (2001) Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 98:4746–4751
Mitkus SN, Hyde TM, Vakkalanka R, Kolachana B, Weinberger DR, Kleinman JE, Lipska BK (2008) Expression of oligodendrocyte-associated genes in dorsolateral prefrontal cortex of patients with schizophrenia. Schizophr Res 98:129–138
Takahashi N, Sakurai T (2013) Roles of glial cells in schizophrenia: possible targets for therapeutic approaches. Neurobiol Dis 53:49–60
Saia-Cereda VM, Cassoli JS, Schmitt A, Falkai P, Nascimento JM, Martins-de-Souza D (2015) Proteomics of the corpus callosum unravel pivotal players in the dysfunction of cell signaling, structure, and myelination in schizophrenia brains. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-015-0621-1
Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, Haroutunian V (2008) Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology 33:2993–3009
Ratta-Apha W, Hishimoto A, Mouri K, Shiroiwa K, Sasada T, Yoshida M, Supriyanto I, Ueno Y, Asano M, Shirakawa O, Togashi H, Takai Y, Sora I (2013) Association analysis of the DISC1 gene with schizophrenia in the Japanese population and DISC1 immunoreactivity in the postmortem brain. Neurosci Res 77:222–227
Trossbach SV, Fehsel K, Henning U, Winterer G, Luckhaus C, Schäble S, Silva MA, Korth C (2014) Peripheral DISC1 protein levels as a trait marker for schizophrenia and modulating effects of nicotine. Behav Brain Res 275:176–182
Nakata K, Lipska BK, Hyde TM, Ye T, Newburn EN, Morita Y, Vakkalanka R, Barenboim M, Sei Y, Weinberger DR, Kleinman JE (2009) DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc Natl Acad Sci USA 106:15873–15878
Oliney A, House R, Gao B, Recksiek P, Phang TL, Sullivan B, Hollis JP, Hopkins J, Shade T, Edwards MG, Vianzon R, Griffith C, Ceilley J, Helfrisch RW, Ritvo Weis E, Weiss D, Gault J (2011) Elevated DISC1 transcript levels in PBMs during acute psychosis in patients with schizophrenia. Transl Biomed 2(1):9
Santoro ML, Gadelha A, Ota VK, Cunha GR, Asevedo E, Noto CS, Spindola LM, Pan PM, Talarico F, Mansur RB, Silva PN, Brietzke E, Cordeiro Q, Bressan RA, Belangero SI (2015) Gene expression analysis in blood of ultra-high risk subjects compared to first-episode of psychosis patients and controls. World J Biol Psychiatry 18:1–6 (Epub ahead of print)
Soares DC, Carlyle BC, Bradshaw NJ, Porteous DJ (2011) DISC1: structure, function, and therapeutic potential for major mental illness. ACS Chem Neurosci 2:609–632
Bernstein HG, Stürze E, Bogerts B (2010) Cell cycle disturbances in schizophrenia: the journey so far. Acta Clin Croat 49(Suppl 1):33–35
Ren Y, Wang Y, Xiao L (2013) Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol 16:691–700
Chiba S, Hashimoto R, Hattori S, Yohda M, Lipska B, Weinberger DR, Kunugi H (2006) Effect of antipsychotic drugs on DISC1 and dysbindin expression in mouse frontal cortex and hippocampus. J Neural Transm 113:1337–1346
Nagai T, Kitahara Y, Ibi D, Nabeshima T, Sawa A, Yamada K (2011) Effects of antipsychotics on the behavioral deficits in human dominant-negative DISC1 transgenic mice with neonatal poly I: c treatment. Behav Brain Res 225:305–310
Mai JK, Paxinos G, Voss T (1997) Atlas of the Human Brain. Academic Press, New York
Acknowledgments
We are grateful to Bianca Jerzykiewicz and Gabriele Meyer-Lotz for excellent technical assistance.
Author Contributions
H-G. B. analyzed the data, researched, wrote, and edited the manuscript. E. J. analyzed the data. H. D. carried out statistical calculations and contributed to photography. C. M. carried out neuropathological studies. J. S wrote and edited the manuscript. B. B. wrote and edited the manuscript.
Role of the funding source
The study was funded by the Otto-von-Guericke-University Magdeburg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bernstein, HG., Jauch, E., Dobrowolny, H. et al. Increased density of DISC1-immunoreactive oligodendroglial cells in fronto-parietal white matter of patients with paranoid schizophrenia. Eur Arch Psychiatry Clin Neurosci 266, 495–504 (2016). https://doi.org/10.1007/s00406-015-0640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-015-0640-y