Abstract
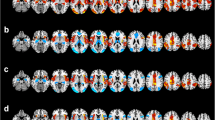
Patients suffering from bipolar affective disorder show deficits in working memory functions. In a previous functional magnetic resonance imaging study, we observed an abnormal hyperactivity of the amygdala in bipolar patients during articulatory rehearsal in verbal working memory. In the present study, we investigated the dynamic neurofunctional interactions between the right amygdala and the brain systems that underlie verbal working memory in both bipolar patients and healthy controls. In total, 18 euthymic bipolar patients and 18 healthy controls performed a modified version of the Sternberg item-recognition (working memory) task. We used the psychophysiological interaction approach in order to assess functional connectivity between the right amygdala and the brain regions involved in verbal working memory. In healthy subjects, we found significant negative functional interactions between the right amygdala and multiple cortical brain areas involved in verbal working memory. In comparison with the healthy control subjects, bipolar patients exhibited significantly reduced functional interactions of the right amygdala particularly with the right-hemispheric, i.e., ipsilateral, cortical regions supporting verbal working memory. Together with our previous finding of amygdala hyperactivity in bipolar patients during verbal rehearsal, the present results suggest that a disturbed right-hemispheric “cognitive–emotional” interaction between the amygdala and cortical brain regions underlying working memory may be responsible for amygdala hyperactivation and affects verbal working memory (deficits) in bipolar patients.



Similar content being viewed by others
References
Phillips ML, Vieta E (2007) Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull 33:893–904. doi:10.1093/schbul/sbm060
Wang F, Kalmar JH, He Y et al (2009) Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 66:516–521. doi:10.1016/j.biopsych.2009.03.023
Usher J, Leucht S, Falkai P, Scherk H (2010) Correlation between amygdala volume and age in bipolar disorder—a systematic review and meta-analysis of structural MRI studies. Psychiatry Res 182:1–8. doi:10.1016/j.pscychresns.2009.09.004
Yurgelun-Todd DA, Gruber SA, Kanayama G et al (2000) fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2:237–248
Lawrence NS, Williams AM, Surguladze S et al (2004) Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55:578–587. doi:10.1016/j.biopsych.2003.11.017
Malhi GS, Lagopoulos J, Ward PB et al (2004) Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci 19:741–754
Rich BA, Vinton DT, Roberson-Nay R et al (2006) Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA 103:8900–8905. doi:10.1073/pnas.0603246103
Pavuluri MN, O’Connor MM, Harral E, Sweeney JA (2007) Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 62:158–167. doi:10.1016/j.biopsych.2006.07.011
Bermpohl F et al (2009) A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disord 11(1):70–75. doi:10.1111/j.1399-5618.2008.00648.x
Blumberg HP, Martin A, Kaufman J et al (2003) Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 160:1345–1347
Blumberg HP, Leung H-C, Skudlarski P et al (2003) A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 60:601–609. doi:10.1001/archpsyc.60.6.601
Gruber SA, Rogowska J, Yurgelun-Todd DA (2004) Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord 82:191–201. doi:10.1016/j.jad.2003.10.010
MacQueen GM, Hajek T, Alda M (2005) The phenotypes of bipolar disorder: relevance for genetic investigations. Mol Psychiatry 10:811–826. doi:10.1038/sj.mp.4001701
Kronhaus DM, Lawrence NS, Williams AM et al (2006) Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord 8:28–39. doi:10.1111/j.1399-5618.2006.00282.x
Melcher T, Wolter S, Falck S et al (2013) Common and disease-specific dysfunctions of brain systems underlying attentional and executive control in schizophrenia and bipolar disorder. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-013-0445-9
Gruber O, von Cramon DY (2001) Domain-specific distribution of working memory processes along human prefrontal and parietal cortices: a functional magnetic resonance imaging study. Neurosci Lett 297:29–32
Gruber O, von Cramon DY (2003) The functional neuroanatomy of human working memory revisited. Evidence from 3-T fMRI studies using classical domain-specific interference tasks. NeuroImage 19:797–809
Amaral DG, Price JL (1984) Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230:465–496. doi:10.1002/cne.902300402
Barbas H, De Olmos J (1990) Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol 300:549–571. doi:10.1002/cne.903000409
Stefanacci L, Suzuki WA, Amaral DG (1996) Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol 375:552–582. doi:10.1002/(SICI)1096-9861(19961125)375:4<552:AID-CNE2>3.0.CO;2-0
Ghashghaei HT, Barbas H (2002) Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115:1261–1279
Stein JL, Wiedholz LM, Bassett DS et al (2007) A validated network of effective amygdala connectivity. NeuroImage 36:736–745. doi:10.1016/j.neuroimage.2007.03.022
Rolls ET (2005) Emotion explained. University Press, Oxford
Dannlowski U et al (2007) Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res 154(1):13–20
Kim MJ, Loucks RA, Palmer AL et al (2011) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410. doi:10.1016/j.bbr.2011.04.025
Ochsner KN, Ray RD, Cooper JC et al (2004) For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 23:483–499. doi:10.1016/j.neuroimage.2004.06.030
Weinberger NA, Metherate R, McKenna T et al (1990) Neural adaptive information processing: a preliminary model of receptive-field plasticity in auditory cortex during Pavlovian conditioning. In: Gabriel M, Moore J (eds) Learning and computational neuroscience: foundations of adaptive networks. The MIT Press, Cambridge, pp 91–138
Pare D, Quirk GJ, Ledoux JE (2004) New vistas on amygdala networks in conditioned fear. J Neurophysiol 92:1–9
Phelps EA, LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48:175–187
Gruber O, Tost H, Henseler I et al (2010) Pathological amygdala activation during working memory performance: evidence for a pathophysiological trait marker in bipolar affective disorder. Hum Brain Mapp 31:115–125. doi:10.1002/hbm.20849
Trost S, Gruber O (2012) Evidence for a double dissociation of articulatory rehearsal and non-articulatory maintenance of phonological information in human verbal working memory. Neuropsychobiology 65:133–140. doi:10.1159/000332335
Gruber O (2001) Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cereb Cortex 11(11):1047–1055. doi:10.1093/cercor/11.11.1047
Friston KJ, Buechel C, Fink GR et al (1997) Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 6:218–229. doi:10.1006/nimg.1997.0291
Penny WD, Holmes AJ (2003) Random-effects analysis. Academic Press, San Diego
Pessoa L (2008) On the relationship between emotion and cognition. Nat Rev Neurosci 9:148–158. doi:10.1038/nrn2317
McIntyre CK, Marriott LK, Gold PE (2003) Cooperation between memory systems: acetylcholine release in the amygdala correlates positively with good performance on a hippocampus-dependent task. Behav Neurosci 117:320–326
Curcić-Blake B, Swart M, Aleman A (2012) Bidirectional information flow in frontoamygdalar circuits in humans: a dynamic causal modeling study of emotional associative learning. Cereb Cortex 22(2):436–445. doi:10.1093/cercor/bhr124
Banks SJ, Eddy KT, Angstadt M et al (2007) Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. doi:10.1093/scan/nsm029
Moses-Kolko EL, Perlman SB, Wisner KL et al (2010) Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry 167:1373–1380. doi:10.1176/appi.ajp.2010.09081235
Versace A, Thompson WK, Zhou D et al (2010) Abnormal left and right amygdala–orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry 67:422–431. doi:10.1016/j.biopsych.2009.11.025
Anand A, Li Y, Wang Y et al (2009) Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res 171:189–198. doi:10.1016/j.pscychresns.2008.03.012
Rosenkranz JA, Moore H, Grace AA (2003) The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci Off J Soc Neurosci 23:11054–11064
Chepenik LG, Raffo M, Hampson M et al (2010) Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res 182:207–210. doi:10.1016/j.pscychresns.2010.04.002
Foland LC, Altshuler LL, Bookheimer SY et al (2008) Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res 162:27–37. doi:10.1016/j.pscychresns.2007.04.007
Gray JR (2001) Emotional modulation of cognitive control: approach-withdrawal states double-dissociate spatial from verbal two-back task performance. J Exp Psychol Gen 130:436–452
Northoff G, Heinzel A, Bermpohl F et al (2004) Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional–cognitive interaction. Hum Brain Mapp 21:202–212. doi:10.1002/hbm.20002
Dolcos F, McCarthy G (2006) Brain systems mediating cognitive interference by emotional distraction. J Neurosci Off J Soc Neurosci 26:2072–2079. doi:10.1523/JNEUROSCI.5042-05.2006
Melcher T, Born C, Gruber O (2011) How negative affect influences neural control processes underlying the resolution of cognitive interference: an event-related fMRI study. Neurosci Res 70:415–427. doi:10.1016/j.neures.2011.05.007
Foland LC, Altshuler LL, Sugar CA, Lee AD et al (2008) Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. NeuroReport 19:221–224
Haldane M, Jogia J, Cobb A, Kozuch E, Kumari V, Frangou S (2008) Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with Lamotrigine monotherapy. Eur Neuropsychopharmacol 18:48–54
Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML (2012) Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord 14:375–410
Lindquist MA, Wager TD (2008) Spatial smoothing in fMRI using prolate spheroidal wave functions. Hum Brain Mapp 29:1276–1287
Sacchet MD, Knutson B (2012) Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage 66C:270–277
Dagli MS, Ingeholm JE, Haxby JV (1999) Localization of cardiac-induced signal change in fMRI. Neuroimage 9:407–415
Windischberger C, Langenberger H, Sycha T, Tschernko EM, Fuchsjager-Mayerl G, Schmetterer L, Moser E (2002) On the origin of respiratory artifacts in BOLD-EPI of the human brain. Magn Reson Imaging 20:575–582
Acknowledgments
This work was partially supported by the Deutsche Forschungsgemeinschaft (DFG) via the Clinical Research Group 241 “Genotype-phenotype relationships and neurobiology of the longitudinal course of psychosis” (http://www.kfo241.de; Grant Number GR 1950/5-1) to O.G..
Conflicts of interest
O.G. was honorary speaker for the following companies: Astra Zeneca, Bristol Myers Squibb, Janssen Cilag, Lilly, Otsuka and Servier. He has been invited to scientific congresses by Astra Zeneca, Janssen Cilag and Pfizer and has received a research grant from Servier. O.G. reports that these potential conflicts have no relation to the subject of the present study. All other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Katharina Stegmayer and Juliana Usher contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stegmayer, K., Usher, J., Trost, S. et al. Disturbed cortico–amygdalar functional connectivity as pathophysiological correlate of working memory deficits in bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci 265, 303–311 (2015). https://doi.org/10.1007/s00406-014-0517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-014-0517-5