Abstract
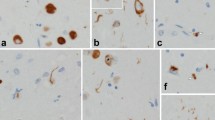
Hippocampal sclerosis (HpScl) is frequent in frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP), but it also occurs in dementia of the elderly with or without accompanying Alzheimer type pathology. HpScl has been hypothesized to be a neurodegenerative process given its association with TDP-43 pathology, but this is still controversial. TDP-43 pathology is found in Lewy body disease (LBD), but no study has focused on the pathologic and genetic characteristics of HpScl in LBD. We found HpScl in 5.2 % of 669 LBD cases (289 transitional and 380 diffuse). Older age, higher Braak neurofibrillary tangle (NFT) stage, and presence of TDP-43 pathology were associated with HpScl. There was no difference in the frequency of HpScl between transitional and diffuse LBD, suggesting that Lewy-related pathology appears to have no direct association with HpScl. All HpScl cases had TDP-43 pathology consistent with Type A pattern. HpScl cases harbored genetic variation in TMEM106B that has been previously associated with FTLD-TDP. Interestingly, the severity of TDP-43-positive fine neurites in CA1 sector, a possible pathologic precursor of HpScl, was associated with the TMEM106B variant. These results demonstrate HpScl in LBD is a TDP-43 proteinopathy and is similar to FTLD-TDP Type A. Furthermore, a subset of LBD cases without HpScl (“pre-HpScl”) had similar pathologic and genetic characteristics to typical HpScl, suggesting that the spectrum of HpScl pathology may be wider than previously thought. Some cases with many extracellular NFTs also had a similar profile. We suggest that HpScl is “masked” in these cases.




Similar content being viewed by others
References
Ala TA, Beh GO, Frey WH 2nd (2000) Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology 54:843–848
Amador-Ortiz C, Lin WL, Ahmed Z et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Arai T, Mackenzie IR, Hasegawa M et al (2009) Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 117:125–136
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Dickson DW, Baker M, Rademakers R (2010) Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis 7:170–174
Dickson DW, Davies P, Bevona C et al (1994) Hippocampal sclerosis: a common pathological feature of dementia in very old (> or =80 years of age) humans. Acta Neuropathol 88:212–221
Finch N, Carrasquillo MM, Baker M et al (2011) TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology 76:467–474
Hatanpaa KJ, Bigio EH, Cairns NJ et al (2008) TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 67:271–279
Higashi S, Iseki E, Yamamoto R et al (2007) Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294
Josephs KA, Ahmed Z, Katsuse O et al (2007) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 66:142–151
Josephs KA, Stroh A, Dugger B, Dickson DW (2009) Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 118:349–358
Josephs KA, Whitwell JL, Weigand SD et al (2014) TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 127:811–824
Kosaka K (2000) Diffuse Lewy body disease. Neuropathology 20(Suppl):S73–S78
Kosaka K (2014) Latest concept of Lewy body disease. Psychiatry Clin Neurosci 68:391–394
Lin WL, Castanedes-Casey M, Dickson DW (2009) Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 68:1167–1176
Mackenzie IR (2007) The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol 114:49–54
Mackenzie IR, Baker M, Pickering-Brown S et al (2006) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090
Mackenzie IR, Neumann M, Baborie A et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113
McKeith IG, Dickson DW, Lowe J et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
Murray ME, Bieniek KF, Banks Greenberg M et al (2013) Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72. Acta Neuropathol 126:545–554
Murray ME, Cannon A, Graff-Radford NR et al (2014) Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol 128:411–421
Nakashima-Yasuda H, Uryu K, Robinson J et al (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229
Nelson PT, Estus S, Abner EL et al (2014) ABCC9 gene polymorphism is associated with hippocampal sclerosis of aging pathology. Acta Neuropathol 127:825–843
Nelson PT, Schmitt FA, Lin Y et al (2011) Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 134:1506–1518
Nelson PT, Smith CD, Abner EL et al (2013) Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 126:161–177
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Pao WC, Dickson DW, Crook JE, Finch NA, Rademakers R, Graff-Radford NR (2011) Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord 25:364–368
Rademakers R, Eriksen JL, Baker M et al (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642
Rutherford NJ, Carrasquillo MM, Li M et al (2012) TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology 79:717–718
Yokota O, Davidson Y, Arai T et al (2010) Effect of topographical distribution of alpha-synuclein pathology on TDP-43 accumulation in Lewy body disease. Acta Neuropathol 120:789–801
Zarow C, Sitzer TE, Chui HC (2008) Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep 8:363–370
Zhang YJ, Xu YF, Cook C et al (2009) Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci USA 106:7607–7612
Acknowledgments
We are grateful to all patients, family members, and caregivers who agreed to brain donation; without their donation these studies would have been impossible. We also acknowledge expert technical assistance of Linda Rousseau and Virginia Phillips for histology and Monica Castanedes-Casey for immunohistochemistry. This research was supported in part by a research Grant from the Uehara Memorial Foundation, The Mangurian Foundation, and NIH Grant P50NS72187, P50AG16574, and R01NS076471.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aoki, N., Murray, M.E., Ogaki, K. et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol 129, 53–64 (2015). https://doi.org/10.1007/s00401-014-1358-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1358-z