Abstract
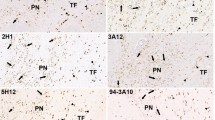
Amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS/PDC) is a progressive neurodegenerative disease affecting the indigenous Chamorro population of Guam. Neuropathologically, PDC is characterized by neuronal loss in the substantia nigra pars compacta with severe widespread neurofibrillary tangles (NFTs) similar to those observed in Alzheimer’s disease (AD), and is thus considered a tauopathy. Following reports of α-synuclein pathology in PDC patients of Guam, PDC has also been neuropathologically classified as a synucleinopathy. Recently, the presence of α-synuclein-positive bodies has been reported in the cerebellum of some patients with Parkinson’s disease (PD), diffuse Lewy body disease (DLBD), or multiple system atrophy (MSA). Using immunohistochemical techniques, we investigated the deposition of α-synuclein in the cerebellum of Guamanian PDC patients. Numerous α-synuclein-immunoreactive spherical structures were found in the molecular layer of the cerebellum of 63.6% of PDC patients. These structures were only seen in patients showing α-synuclein pathology in the amygdala. The average density of α-synuclein-immunoreactive structures in the cerebellum of Guamanian PDC patients was almost an order of magnitude higher than in non-Guamanian PD patients, and this α-synuclein pathology was much more pronounced in the hemisphere than in the vermis. In addition, double immunohistochemistry revealed that cerebellar α-synuclein is co-localized with the neuronal marker calbindin and with glial-fibrillary acidic protein, suggesting the involvement of Purkinje cells and Bergmann glia. These findings demonstrate that the α-synuclein pathology in PDC of Guam affects not only the amygdala, but also the cerebellum, where it appears to involve both Purkinje cells and specialized astrocytes.




Similar content being viewed by others
References
Arai Y, Yamazaki M, Mori O, Muramatsu H, Asano G, Katayama Y (2001) Alpha-synuclein-positive structures in cases with sporadic Alzheimer’s disease: morphology and its relationship to tau aggregation. Brain Res 888:287–296
Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Ueda K, Ikeda K, Kawai M (1999) Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson’s disease and in dementia with Lewy bodies. Brain Res 843:53–61
Buée-Scherrer V, Buée L, Hof PR, Leveugle B, Gilles C, Loerzel AJ, Perl DP, Delacourte A (1995) Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: immunochemical characterization of tau proteins. Am J Pathol 68:924–932
Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM, Trojanowski JQ (2002) Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam. Am J Pathol 160:1725–1731
Galasko D, Salmon DP, Craig UK, Thal LJ, Schellenberg G, Wiederholt W (2002) Clinical features and changing patterns of neurodegenerative disorders on Guam, 1997–2000. Neurology 58:90–97
Gentleman SM, Perl D, Allsop D, Clinton J, Royston MC, Roberts GW (1991) Beta (A4)-amyloid protein and parkinsonian dementia complex of Guam. Lancet 337:55–56
Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H (1999) Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci 2:139–143
Hayashi S, Akasaki Y, Morimura Y, Takauchi S, Sato M, Miyoshi K (1992) An autopsy case of late infantile and juvenile neuroaxonal dystrophy with diffuse Lewy bodies and neurofibrillary tangles. Clin Neuropathol 11:1–5
Hirano A, Malamud N, Kurland LT (1961) Parkinsonism-dementia complex, an endemic disease on the island of Guam II. Pathological features. Brain 84:662–679
Hirano A, Malamud N, Elizan TS, Kurland LT (1966) Amyotrophic lateral sclerosis and parkinsonism-dementia complex on Guam. Further pathologic studies. Arch Neurol 15:35–51
Hirano A, Arumugasamy N, Zimmerman HM (1967) Amyotrophic lateral sclerosis. A comparison of Guam and classical cases. Arch Neurol 16:357–363
Hof PR, Perl DP, Loerzel AJ, Morrison JH (1991) Neurofibrillary tangle distribution in the cerebral cortex of parkinsonism-dementia cases from Guam: differences with Alzheimer’s disease. Brain Res 564:306–313
Iseki E, Marui W, Kosaka K, Ueda K (1999) Frequent coexistence of Lewy bodies and neurofibrillary tangles in the same neurons of patients with diffuse Lewy body disease. Neurosci Lett 265:9–12
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, deSilva HA, Kittel A, Saitoh T (1995) The precursor protein of non-A beta component of Alzheimer’s disease is a presynaptic protein of the central nervous system. Neuron 14:467–475
Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R (1999) Alpha-synuclein binds to tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem 274:25481–25489
Kuzuhara S, Yoshimura M (1993) Clinical and neuropathological aspects of diffuse Lewy body disease in the elderly. Adv Neurol 60:464–469
Lilienfeld DE, Perl DP, Olanow CW (1994) Guam neurodegeneration. In: Calne DB (ed) Neurodegenerative diseases. Saunders, Philadelphia, pp 895–908
Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, St George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM, Iwatsubo T, Trojanowski JQ (1998) Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153:1365–1370
Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ (1999) Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol 45:353–357
Malamud N, Hirano A, Kurland LT (1961) Pathoanatomic changes in amyotrophic lateral sclerosis on Guam. Neurology 5:401–414
Marui W, Iseki E, Ueda K, Kosaka K (2000) Occurrence of human alpha-synuclein immunoreactive neurons with neurofibrillary tangle formation in the limbic areas of patients with Alzheimer’s disease. J Neurol Sci 174:81–84
Mawal-Dewan M, Schmidt L, Balin B, Perl DP, Lee VMY, Trojanowski JQ (1996) Identification of phosphorylation sites in PHF-tau from patients with Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex. J Neuropathol Exp Neurol 55:1051–1059
Mori F, Piao YS, Hayashi S, Fujiwara H, Hasegawa M, Yoshimoto M, Iwatsubo T, Takahashi H, Wakabayashi K (2003) α-Synuclein accumulates in Purkinje cells in Lewy body disease but not in multiple system atrophy. J Neuropathol Exp Neurol 62:812–819
Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747
Perl DP (2001) Amyotrophic lateral sclerosis/parkinsonism dementia complex of Guam. In: Hof PR, Mobbs CV (eds) Functional neurobiology of aging. Academic Press, San Diego, pp 183–201
Piao YS, Wakabayashi K, Hayashi S, Yoshimoto M, Takahashi H (2000) Aggregation of α-synuclein/NACP in the neuronal and glial cells in diffuse Lewy body disease: a survey of six patients. Clin Neuropathol 19:163–169
Piao YS, Mori F, Hayashi S, Tanji K, Yoshimoto M, Kakita A, Wakabayashi K, Takahashi H (2003) α-Synuclein pathology affecting Bergmann glia of the cerebellum in patients with α-synucleinopathies. Acta Neuropathol (Berl) 105:403–409
Rezaie P, Cairns NJ, Chadwick A, Lantos PL (1996) Lewy bodies are located preferentially in limbic areas in diffuse Lewy body disease. Neurosci Lett 212:111–114
Saito Y, Kawai M, Inoue K, Sasaki R, Arai H, Nanba E, Kuzuhara S, Ihara Y, Kanazawa I, Murayama S (2000) Widespread expression of alpha-synuclein and tau immunoreactivity in Hallervorden-Spatz syndrome with protracted clinical course. J Neurol Sci 177:48–59
Schmidt ML, Martin JA, Lee VM, Trojanowski JQ (1996) Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer’s disease and Lewy body disorders. Acta Neuropathol (Berl) 91:475–481
Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha synuclein in Lewy bodies. Nature 388:232–233
Ventura R, Harris KM (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19:6897–6906
Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H (2000) NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol (Berl) 99:14–20
Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K (2000) Alpha-synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol 59:585–591
Acknowledgements
We thank the Chamorro patients and their families for making this research possible. A. Kapustin, C. Buitron, and W.G.M. Janssen provided expert technical assistance. Supported by NIH grants AG05138 and AG14382 to DPP and PRH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sebeo, J., Hof, P.R. & Perl, D.P. Occurrence of α-synuclein pathology in the cerebellum of Guamanian patients with parkinsonism-dementia complex. Acta Neuropathol 107, 497–503 (2004). https://doi.org/10.1007/s00401-004-0840-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0840-4