Abstract
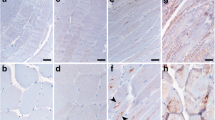
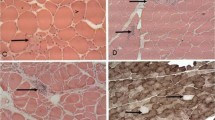
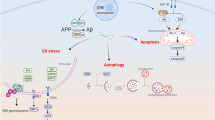
This study was undertaken to determine the C terminus of amyloid β protein (Aβ), accumulated in vacuolated muscle fibers, and compare these findings to the level of oxidative stress. Eight patients with myopathies characterized by rimmed vacuoles (RVs) were analyzed. Monoclonal antibodies specific to Aβ40 or Aβ42(43) revealed that the Aβ42(43) immunoreactivity was solely distributed in the vacuolated muscle fibers, and that only a part was also immunopositive for anti-Aβ40. Quantitative analyses in four specimens, in which eight or more vacuolated muscle fibers were observed, revealed that the mean incidence of Aβ42(43)-positive muscle fibers was 79.5±6.2% in total vacuolated muscle fibers, whereas that of the Aβ40-positive fibers was 42.9±12.6%. The predominance of Aβ42(43) deposition was statistically significant (P<0.05). Aβ deposition was then compared with the distribution of oxidative nucleic acid damage in muscle fibers using a monoclonal antibody against 8-hydroxy-2'-deoxyguanosine and 8-hydroxyguanosine (8OHdG&G). The cytoplasmic staining for anti-8OHdG&G was found not only in vacuolated muscle fibers, but also in other muscle fibers including morphologically normal ones. Positive staining was completely abolished by RNase pretreatment and, thus, was suggested to reflect an increase of cellular RNA oxidation. The distribution of 8OHdG&G was much broader than the Aβ deposition. These data suggest that Aβ42(43) is predominantly involved in the pathogenesis of muscle fiber degeneration with RVs, and that oxidative damage may precede Aβ deposition in muscle fibers and play a key role in the pathomechanism of myopathies with RVs.



Similar content being viewed by others
References
Antunes F, Cadenas E, Brunk UT (2001) Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J 356:549–555
Askanas V, Engel WK (1995) New advances in the understanding of sporadic inclusion-body myositis and hereditary inclusion-body myopathies. Curr Opin Rheumatol 7:486–496
Askanas V, Engel WK, Alvarez RB (1992) Light and electron microscopic localization of β-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol 141:31–36
Banwell BL Engel AG (2000) αB-crystallin immunolocalization yields new insights into inclusion body myositis. Neurology 54:1033–1041
Brais B, Bouchard JP, Xie YG, Rochefort DL, Chrétien N, Tomé FMS, Lafrenière RG, Rommens JM, Uyama E, Nohira O, Blumen S, Korczyn AD, Heutink P, Mathieu J, Duranceau A, Codère F, Fardeau M, Rouleau GA (1998) Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet 18:164–167
Ditaranto K, Tekirian TL, Yang AJ (2001) Lysosomal membrane damage in soluble Aβ-mediated cell death in Alzheimer's disease. Neurobiol Dis 8:19–31
Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sedeh M, Grabov-Nardini G, Shmilevich I, Friedmann A, Karpati G, Bradley WG, Baumbach L, Lancet D, Asher EB, Beckmann JS, Argov Z, Mitrani-Rosenbaum S (2001) The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet 29:83–87
Fukuchi K, Pham D, Hart M, Li L, Lindsey JR (1998) Amyloid-β deposition in skeletal muscle of transgenic mice. Am J Pathol 153:1687–1693
Goldbaum O, Richter-Landsberg C (2001) Stress proteins in oligodendrocytes: differential effects of heat shock and oxidative stress. J Neurochem 78:1233–1242
Ii K, Hizawa K, Nonaka I, Sugita H, Kominami E, Katunuma N (1986) Abnormal increases of lysosomal cysteinine proteinases in rimmed vacuoles in the skeletal muscle. Am J Pathol 122:193–198
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y (1994) Visualization of Aβ 42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: Evidence that an initially deposited species is Aβ42(43). Neuron 13:45–53
Jarrett JT, Lansbury PT Jr (1993) Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73:1055-1058
Jin L-W, Hearn MG, Ogburn CE, Dang N, Nochlin D, Ladiges WC, Martin GM (1998) Transgenic mice over-expressing the C-99 fragment of βPP with an α-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol 153:1679–1686
Kumamoto T, Ito T, Horinouchi H, Ueyama H, Toyoshima I, Tsuda T (2000) Increased lysosome-related proteins in the skeletal muscles of distal myopathy with rimmed vacuoles. Muscle Nerve 23:1686–1693
Misonou H, Morishima-Kawashima M, Ihara Y (2000) Oxidative stress induces intracellular accumulation of amyloid β-protein (Aβ) in human neuroblastoma cells. Biochem 39:6951–6959
Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, Smith MA (2000) Neuronal oxidative stress precedes amyloid-β deposition in Down syndrome. J Neuropathol Exp Neurol 59:1011–1017
Oyama F. Murakami N, Ihara Y (1998) Chloroquine myopathy suggests that tau is degraded in lysosomes: implication for the formation of paired helical filaments in Alzheimer's disease. Neurosci Res (Suppl) 31:1–8
Querfurth HW, Suhara T, Rosen KM, McPhie DL, Fujio Y, Tejada G, Neve RL, Adelman LS, Walsh K (2001) β-Amyloid peptide expression is sufficient for myotube death: implications for human inclusion body myopathy. Mol Cell Neurosci 17:793–810
Schultz C, Dick EJ Jr, Cox AB, Hubbard GB, Braak E, Braak H (2001) Expression of stress proteins alpha B-crystallin, ubiquitin, and hsp27 in pallido-nigral spheroids of aged rhesus monkeys. Neurobiol Aging 22:677–682
Tateyama M, Nagano I, Yoshioka M, Chida K, Nakamura S, Itoyama Y (1997) Expression of tumor necrosis factor -α in muscles of polymyositis. J Neurol Sci 146:45–51
Tateyama M, Tobita M, Takeda A, Chida K, Onodera Y, Kikuchi A, Aoyagi N, Itoyama Y (2000) DNA single-strand breaks are increased in muscle diseases with rimmed vacuoles. Acta Neuropathol 100:390–394
Tateyama M, Fujihara K, Ishii N, Sugamura K, Onodera Y, Itoyama Y (2002) Expression of OX40 in muscles of polymyositis and granulomatous myopathy. J Neurol Sci 194:29–34
Tsuruta Y, Furuta A, Taniguchi N, Yamada T, Kira J, Iwaki T (2002) Increased expression of manganese superoxide dismutase is associated with that of nitrotyrosine in myopathies with rimmed vacuoles. Acta Neuropathol 103:59–65
Tsuzuki K, Fukatsu R, Takamaru Y, Yoshida T, Hayashi Y, Yamaguchi H, Fujii N, Takahata N (1995) Amyloid β protein in rat soleus muscle in chloroquine-induced myopathy using end-specific antibodies for Aβ40 and Aβ42: immunohistochemical evidence for amyloid β protein. Neurosci lett 202:77–80
Yan C, Ikezoe K, Nonaka I (2001) Apoptotic muscle fiber degeneration in distal myopathy with rimmed vacuoles. Acta Neuropathol 101:9–16
Acknowledgement
We wish to thank to Dr. T. Iwatsubo for informative comments. This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Health, Labor and Welfare (A.T.). We are grateful to Mr. Brent Bell for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tateyama, M., Takeda, A., Onodera, Y. et al. Oxidative stress and predominant Aβ42(43) deposition in myopathies with rimmed vacuoles. Acta Neuropathol 105, 581–585 (2003). https://doi.org/10.1007/s00401-003-0685-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-003-0685-2