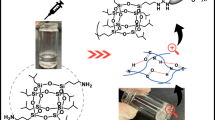
Abstract
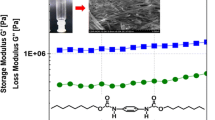
A synthesis strategy for low molecular weight organogelators using the ureidopyrimidinone (UPy) group is reported. The prepared gelators showed robust thermal reversible gelation abilities in various solvents, including dimethyl sulfoxide. The morphology of the dried gels was determined using scanning electron microscopy, revealing a macroscopic porous structure of the gels. Rheology was performed to determine storage (G′) and loss modulus (G″) confirming network gel structures.





Similar content being viewed by others
References
Abdallah DJ, Weiss RG (2000) Organogels and low molecular mass organic gelators. Adv Mater 12:1237–1247
Terech P, Weiss RG (1997) Low molecular mass gelators of organic liquids and the properties of their gels. Chem Rev 97:3133–3159
Sangeetha NM, Maitra U (2005) Supramolecular gels: functions and uses. Chem Soc Rev 34:821–836
Buerkle LE, Rowan SJ (2012) Supramolecular gels formed from multi-component low molecular weight species. Chem Soc Rev 41:6089–6102
Smith DK (2006) Dendritic supermolecules – towards controllable nanomaterials. Chem Commun 7:34–44
Sivakova S, Rowan SJ (2005) Nucleobases as supramolecular motifs. Chem Soc Rev 34:9–21
Piepenbrock MOM, Lloyd GO, Clarke N, Steed JW (2010) Metal-and anion-binding supramolecular gels. Chem Rev 110:1960–2004
Suzuki M, Hanabusa K (2009) L-Lysine-based low-molecular-weight gelators. Chem Soc Rev 38:967–975
Rubio J, Martí-Centelles V, Burguete MI, Luis SV (2013) Synthesis and organogelating ability of bis-urea pseudopeptidic compounds. Tetrahedron 69:2302–2308
Simeone L, Milano D, De Napoli L, Irace C, Di Pascale A, Boccalon M, Tecilla P, Montesarchio D (2011) Design, synthesis and characterisation of guanosine-based amphiphiles. Chem Eur J 17:13854–13865
Wang X, Zhou L, Wang H, Luo Q, Xu J, Liu J (2011) Reversible organogels triggered by dynamic K + binding and release. J Colloid Interface Sci 353:412–419
Du P, Wang G, Zhao X, Li G, Jiang X, Li Z (2010) Two novel quadruple hydrogen-bonding motifs: the formation of supramolecular polymers, vesicles, and organogels. Tetrahedron Lett 51:188–191
Beijer FH, Sijbesma RP, Kooijman H, Spek AL, Meijer EW (1998) Strong dimerization of ureidopyrimidones via quadruple hydrogen bonding. J Am Chem Soc 120:6761–6769
Han JT, Lee DH, Ryu CY, Cho K (2004) Fabrication of superhydrophobic surface from a supramolecular organosilane with quadruple hydrogen bonding. J Am Chem Soc 126:4796–4797
Chen Y, Ballard N, Gayet F, Bon SAF (2012) High internal phase emulsion gels (HIPE-gels) from polymer dispersions reinforced with quadruple hydrogen bond functionality. Chem Commun 48:1117–1119
Chen Y, Ballard N, Bon SAF (2013) Moldable high internal phase emulsion hydrogel objects from non-covalently crosslinked poly(N-isopropylacrylamide) nanogel dispersions. Chem Commun 49:1524–1526
Chen X, Ayres N (2010) Synthesis of novel polymer/urea peptoid conjugates using RAFT polymerization. Macromolecules 43:1341–1348
Chen X, Ayres N (2011) Synthesis of low grafting density molecular brush from a poly(N-alkyl urea peptoid) backbone. J Polym Sci Polym Chem 49:3030–3037
Chen X, Ding K, Ayres N (2011) Investigation into fiber formation in N-alkyl urea peptoid oligomers and the synthesis of a water-soluble PEG/N-alkyl urea peptoid oligomer conjugate. Polym Chem 2:2635–2642
Taylor L, Chen X, Ding K, Ayres N (2013) Synthesis of a glycosaminoglycan polymer mimetic using an N-alkyl-N, N-linked urea oligomer containing glucose pendant groups. Polym Int. doi:10.1002/pi.4567
Folmer BJB, Sijbesma RP, Versteegen RM, van der Rijt JAJ, Meijer EW (2000) Supramolecular polymer materials: chain extension of telechelic polymers using a reactive hydrogen-bonding synthon. Adv Mater 12:874–878
Ligthart GBWL, Ohkawa H, Sijbesma RP, Meijer EW (2006) Pd-catalyzed amidation of 2-chloro-and 2,7-dichloro-1,8-naphthyridines. J Org Chem 71:375–378
Ajayaghosh A, Praveen VK, Vijayakumar C (2008) Organogels as scaffolds for excitation energy transfer and light harvesting. Chem Soc Rev 37:109–122
Wang C, Wang Z, Zhang D, Zhu D (2006) Thermal modulation of the monomer/excimer fluorescence for bispyrene molecules through the gel-solution transition of an organogel: a thermo-driven molecular fluorescence switch. Chem Phys Lett 428:130–133
Winnik FM (1993) Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem Rev 93:587–614
Østergaard ME, Hrdlicka PJ (2011) Pyrene-functionalized oligonucleotides and locked nucleic acids (LNAs): tools for fundamental research, diagnostics, and nanotechnology. Chem Soc Rev 40:5771–5788
Hardy JG, Hirst AR, Smith DK, Brennan C, Ashworth I (2005) Controlling the materials properties and nanostructure of a single-component dendritic gel by adding a second component. Chem Commun 3:385–387
Brinker C, Scherer G (1990) Sol–gel Science: The Physics and Chemistry of Sol–gel Processing. Academic Press, San Diego, CA
Hirst AR, Coates IA, Boucheteau TR, Miravet JF, Escuder B, Castelletto V, Hamley IW, Smith DK (2008) Low-molecular-weight gelators: elucidating the principles of gelation based on gelator solubility and a cooperative self-assembly model. J Am Chem Soc 130:9113–9121
Lescanne M, Colin A, Mondain-Monval O, Fages F, Pozzo JL (2003) Structural aspects of the gelation process observed with low molecular mass organogelators. Langmuir 19:2013–2020
Li JL, Yuan B, Liu XY, Xu HY (2010) Cryst Growth Design 10:2699–2706
Raghavan SR (2009) Distinct character of surfactant gels: a smooth progression from micelles to fibrillar networks. Langmuir 25:8382–8385
Zhu G, Dordick JS (2006) Solvent effect on organogel formation by low molecular weight molecules. Chem Mater 18:5988–5995
Feng L, Cavicchi KA (2012) Investigation of the relationships between the thermodynamic phase behavior and gelation behavior of a series of tripodal trisamide compounds. Soft Matter 8:6483–6492
Gao J, Wu S, Rogers MA (2012) Harnessing Hansen solubility parameters to predict organogel formation. J Mater Chem 22:12651–12658
Raynal M, Bouteiller L (2011) Organogel formation rationalized by Hansen solubility parameters. Chem Commun 47:8271–8273
Elkins CL, Park T, McKee MG, Long TE (2005) Synthesis and characterization of poly(2-ethylhexyl methacrylate) copolymers containing pendant, self-complementary multiple-hydrogen-bonding sites. J Polym Sci Part A Polym Chem 43:4618–4631
McKee MG, Elkins CL, Park T, Long TE (2005) Influence of random branching on multiple hydrogen bonding in poly(alkyl methacrylate)s. Macromolecules 38:6015–6023
Brunsveld L, Folmer BJB, Meijer EW, Sijbesma RP (2001) Supramolecular polymers. Chem Rev 101:4071–4098
Sijbesma RP, Meijer EW (2003) Quadruple hydrogen bonded systems. Chem Commun 1:5–16
Wang XW, Li XQ, Shao XB, Zhao X, Deng P, Jiang XK, Li ZT, Chen GJ (2003) Selective rearrangements of quadruply hydrogen-bonded dimer driven by donor–acceptor interaction. Chem Eur J 9:2904–2913
Acknowledgments
Acknowledgment is made to the donors of the American Chemical Society Petroleum Research Fund (51850-DN17) and The University of Cincinnati for support of this research for N.A. and X.C. P.F. and K.A.C. thank The National Science Foundation (NSF-CHE 1012237).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 9783 kb)
Rights and permissions
About this article
Cite this article
Chen, X., Fei, P., Cavicchi, K.A. et al. The poor solubility of ureidopyrimidone can be used to form gels of low molecular weight N-alkyl urea oligomers in organic solvents. Colloid Polym Sci 292, 477–484 (2014). https://doi.org/10.1007/s00396-013-3087-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-3087-6