Abstract
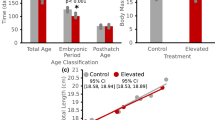
Cetaceans (dolphins and whales) are born into the aquatic environment and are immediately challenged by the demands of hypoxia and exercise. This should promote rapid development of the muscle biochemistry that supports diving, but previous research on two odontocete (toothed whales and dolphins) species showed protracted postnatal development for myoglobin content and buffering capacity. A minimum of 1 and 1.5 years were required for Fraser’s (Lagenodelphis hosei) and bottlenose (Tursiops truncatus) dolphins to obtain mature myoglobin contents, respectively; this corresponded to their lengthy 2 and 2.5-year calving intervals (a proxy for the dependency period of cetacean calves). To further examine the correlation between the durations for muscle maturation and maternal dependency, we measured myoglobin content and buffering capacity in the main locomotor muscle (longissimus dorsi) of harbor porpoises (Phocoena phocoena), a species with a comparatively short calving interval (1.5 years). We found that at birth, porpoises had 51 and 69 % of adult levels for myoglobin and buffering capacity, respectively, demonstrating greater muscle maturity at birth than that found previously for neonatal bottlenose dolphins (10 and 65 %, respectively). Porpoises achieved adult levels for myoglobin and buffering capacity by 9–10 months and 2–3 years postpartum, respectively. This muscle maturation occurred at an earlier age than that found previously for the dolphin species. These results support the observation that variability in the duration for muscular development is associated with disparate life history patterns across odontocetes, suggesting that the pace of muscle maturation is not solely influenced by exposure to hypoxia and exercise. Though the mechanism that drives this variability remains unknown, nonetheless, these results highlight the importance of documenting the species-specific physiological development that limits diving capabilities and ultimately defines habitat utilization patterns across age classes.




Similar content being viewed by others
References
Amano M, Miyazaki N, Yanagisawa F (1996) Life history of Fraser’s dolphin, Lagenodelphis hosei, based on a school captured off the Pacific Coast of Japan. Mar Mamm Sci 12(2):199–214
Archer FI, Robertson KM (2004) Age and length at weaning and development of diet of pantropical spotted dolphions Stenella attenuata from the eastern tropical Pacific. Mar Mamm Sci 20:232–245
Bowen WD, Oftedal OT, Boness BJ (1985) Birth to weaning in 4 days: remarkable growth in the hooded seal, Cystophora cristata. Can J Zool 63:2841–2846
Bowen WD, Boness BJ, Oftedal OT (1987) Mass transfer from mother to pup and subsequent mass loss by the weaned pup in the hooded seal, Cystophora cristata. Can J Zool 65:1–8
Bowen WD, Lawson JW, Beck B (1993) Seasonal and geographic variation in the species composition and size of prey consumed by grey seals (Halichoerus grypus) on the Scotian shelf. Can J Fish Aquat Sci 50:1768–1778
Burns JM, Hammill MO (2008) Does iron availability limit oxygen store development in seal pups? In: Proceedings of the 4th CPB meeting in Africa: MARA 2008 “molecules to migration: the pressures of life”, pp 417–428
Burns JM, Costa DP, Frost K, Harvey JT (2005) Development of the body oxygen stores in harbor seals: effects of age, mass, and body composition. Phys Biochem Zool 78(6):1057–1068
Burns JM, Lestyk KC, Folkow LP, Hammill MO, Blix AS (2007) Size and distribution of oxygen stores in harp and hooded seals from birth to maturity. J Comp Physiol B 177:687–700
Castellini MA, Somero GN (1981) Buffering capacity of vertebrate muscle; correlations with potentials for anaerobic function. J Comp Physiol B 143:191–198
Castellini MA, Kooyman GL, Ponganis PJ (1992) Metabolic rates of freely diving weddell seals: correlations with oxygen stores, swim velocity and diving duration. J Exp Biol 165:181–194
Cockcroft VG, Ross JB (1990) Observations on the early development of a captive bottlenose dolphin calf. In: Leatherwood SJ, Reeves R (eds) The bottlenose dolphin. Academic Press, San Diego, pp 461–478
Dolar ML, Suarez P, Ponganis PJ, Kooyman GL (1999) Myoglobin in pelagic small cetaceans. J Exp Biol 202:227–236
Evans PGH (1987) The natural history of whales and dolphins. Christopher Helm Ltd, Bromley
Field IC, Bradshaw CJA, van den Hoff J, Burton HR, Hindel MA (2007) Age-related shifts in the diet composition of southern elephant seals expand overall foraging niche. Mar Biol 150(6):1441–1452
Fowler SL, Costa DP, Arnould JPY, Gales NJ, Burns JM (2007) Ontogeny of oxygen stores and physiological diving capability in Australian sea lions. Funct Ecol 21:922–935
Gaskin DE, Blair BA (1977) Age determination of harbor porpoise, Phocoena phocoena (L.), in the western North Atlantic. Can J Zool 55:18–30
Gearin PJ, Melin SR, Delong RL, Kajimura H, Johnson MA (1994) Harbor porpoise interactions with a Chinook salmon set-net fishery in Washington state. In: Perrin WF, Donovan GP, Barlow J (eds) Gillnets and cetaceans. Report of the International Whaling Commission, special issue 15, pp 427–437
Geiseler SJ, Blix AS, Burns JM, Folkow LP (2013) Rapid postnatal development of myoglobin from large liver iron stores in hooded seals. J Exp Biol 216:1793–1798
Geraci JR, Lounsbury VJ (2005) Marine mammals ashore: a field guide for strandings, 2nd edn. National Aquarium, Baltimore 372 pp
Goforth HW (1986) Glycogenetic responses and force production characteristics of a bottlenose dolphin (Tursiops truncatus) while exercising against a force transducer. Ph.D. thesis, University of California, Los Angeles, p 137
Gore CJ, Hahn AG, Aughey RJ, Martin DT, Ashenden MJ, Clark SA, Garnham AP, Roberts AD, Slater GJ, McKenna MJ (2001) Live high: train low increases muscle buffering capacity and submaximal cycling efficiency. Acta Physiol Scand 173:275–286
Gregory IC, Hulands GH, Millar RA (1972) On the use of a value for haemoglobin oxygen combining capacity in the indirect determination of blood oxygen content in man. Br J Anaesth 44:222
Griffiths RI, Baldwin J, Berger PJ (1994) Metabolic development of the sheep diaphragm during fetal and newborn life. Respir Phys 95:337–347
Haggblom L, Terwilliger RC, Terwilliger NB (1988) Changes in myoglobin and lactate dehydrogenase in muscle tissues of a diving bird, the pigeon guillemot, during maturation. Comp Biochem Physiol B 91(2):273–277
Hochachka PW (1986) Balancing conflicting metabolic demands of exercise and diving. Fed Proc 45:2948–2952
Hochachka PW, Storey KB (1975) Metabolic consequences of diving in animals and man. Science 187:613–621
Jeglinski JWE, Werner C, Robinson PW, Costa DP, Trillmich F (2012) Age, body mass and environmental variation shape the foraging ontogeny of Galapagos sea lions. Mar Ecol Prog Ser 453:279–296
Kanatous SB, Mammen PPA, Rosenberg PB, Martin CM, White MD, DiMaio JM, Huang G, Muallem S, Garry DJ (2009) Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am J Physiol Cell Physiol 296:C393–C402
Kleiber M (1975) The fire of life: an introduction to animal energetics. Robert E. Krieger Publishing Co., New York
Koopman HN, Westgate AJ, Read AJ (1999) Hematology values of wild harbor porpoises (Phocoena phocoena) from the Bay of Fundy, Canada. Mar Mamm Sci 15(1):52–64
Kooyman GL (1989) Diverse divers: physiology and behaviour. Springer, Berlin
Kooyman GL, Ponganis PJ (1998) The physiological basis of diving to depth: birds and mammal. Ann Rev Physiol 60:19–32
Kooyman GL, Wahrenbrock EA, Castellini MA, Davis RW, Sinnett EE (1980) Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: Evidence of preferred pathways from blood chemistry and behavior. J Comp Physiol B 138:335–346
Lenfant C, Johanson K, Torrance JD (1970) Gas transport and oxygen storage capacity in some pinnipeds and the sea otter. Respir Physiol 9:277–286
Lestyk KC, Folkow LP, Blix AS, Hammill MO, Burns JM (2009) Development of myoglobin concentrations and acid buffering capacity in harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals from birth to maturity. J Comp Physiol B 179:985–996
MacArthur RA (1990) Seasonal changes in the oxygen storage capacity and aerobic dive limits of the muskrat (Ondatra zibethicus). J Comp Physiol B 160:593–599
Mann J, Sargeant B (2003) Like mother, like calf: the ontogeny of foraging traditions in wild Indian Ocean bottlenose dolphins (Tursiops sp.). In: Fragaszy DM, Perry S (eds) The biology of traditions. Cambridge University Press, Cambridge, pp 236–266
Mann J, Smuts B (1999) Behavioral development in wild bottlenose dolphin newborns (Tursiops sp.). Behaviour 136:529–566
McLellan WA, Koopman HN, Rommel SA, Read AJ, Potter CW, Nicolas JR, Westgate AJ, Pabst DA (2002) Ontogenetic allometry and body composition of harbour porpoises (Phocoena phocoena, L.) from the western North Atlantic. J Zool Lond 257:457–471
Miles JA, Herzing DL (2003) Underwater analysis of the behavioral development of Atlantic spotted dolphin (Stenella attenuata) calves (birth to 4 years of age). Aquat Mamm 29:363–377
Morrison P, Rosenmann M, Sealander JA (1966) Seasonal variation of myoglobin in the northern red-backed vole. Am J Physiol 211(6):1305–1308
Noren SR (2004) Muscle buffering capacities in cetaceans: Influences of diving performance, swimming performance, body size, and postpartum development. Mar Mamm Sci 20(4):808–822
Noren SR, Williams TM (2000) Body size and skeletal muscle myoglobin of cetaceans: Adaptations for maximizing dive duration. Comp Biochem Physiol A 126:181–191
Noren SR, Williams TM, Pabst DA, McLellan B, Dearolf J (2001) The development of diving in marine endotherms: preparing the skeletal muscles of dolphins, penguins, and seals for activity during submergence. J Comp Physiol B 171:127–134
Noren SR, Iverson SJ, Boness DJ (2005) Development of the blood and muscle oxygen stores in grey seals (Halichoerus grypus): implications for juvenile diving capacity and the necessity of a terrestrial postweaning fast. Physiol Biochem Zool 78(4):482–490
Noren SR, Biedenbach G, Edwards EF (2006) The ontogeny of swim performance and mechanics in bottlenose dolphins (Tursiops truncatus). J Exp Biol 209(23):4724–4731
Noren SR, Biedenbach G, Redfern JV, Edwards EF (2008) Hitching a ride: the formation locomotion strategy of dolphin calves. Funct Ecol 22:278–283
Noren SR, Williams TM, Ramirez K, Boehm J, Glenn M, Cornell L (2012) Changes in partial pressures of respiratory gases during submerged voluntary breath-hold across Odontocetes: Is body mass important? J Comp Physiol B 182(2):299–309
Otani S, Naito Y, Kato A, Kawamura A (2001) Oxygen consumption and swim speed of the harbor porpoise Phocoena phocoena. Fish Sci 67:894–898
Pabst DA (1993) Intramuscular morphology and tendon geometry of the epaxial swimming muscles of dolphins. J Zool Lond 230:159–176
Peddemors VM, Fothergill AW, Cockcroft VG (1992) Feeding and growth in a captive born bottlenose dolphin, Tursiops truncatus S. Afr J Zool 37:74–80
Ponganis PJ (2011) Diving mammals. Compr Phys 1:517–535
Ponganis PJ, Kooyman GL, Castellini MA (1993) Determinants of the aerobic dive limit of Weddell seals: analysis of diving metabolic rates, postdive end tidal PO2’s and blood and muscle oxygen stores. Phys Zool 66:732–749
Ponganis PJ, Kooyman GL, Baranov EA, Thorson PH, Steward BS (1997) The aerobic submersion limit of Baikal seals, Phoca sibirica. Can J Zool 75:1323–1327
Ponganis PJ, Starke LN, Horning M, Kooyman GL (1999) Development of diving capacity in emperor penguins. J Exp Biol 202:781–786
Ponganis PJ, Meir JU, Williams CL (2010) Oxygen store depletion and the aerobic dive limit in emperor penguins. Aquat Biol 8:237–245
Read AJ, Hohn AA (1995) Life in the fast land: the life history of harbor porpoises from the Gulf of Maine. Mar Mamm Sci 11(4):423–440
Read AJ, Wells RS, Hohn AA, Scott MD (1993) Patterns of growth in wild bottlenose dolphins, Turisops truncatus. J Zool Lond 231:107–123
Reed JZ, Chambers C, Hunter CJ, Lockyer C, Kastelein R, Fedak MA, Boutilier RG (2000) Gas exchange and heart rate in the harbour porpoise Phocoena phocoena. J Com???p Physiol B 170:1–10
Reeves RR, Stewart BS, Leatherwood S (1992) The Sierra Handbook of Seals and Sirenians. Sierra Club Books, San Francisco
Reynafarje B (1963) Simplified method for the determination of myoglobin. J Lab Clin Med 61(1):138–145
Richmond JP (2004) Ontogeny of total body oxygen stores and aerobic dive potential in the steller sea lion (Eumetopias jubatus). Masters Thesis, University of Alaska, Anchorage, p 114
Richmond JP, Burns JM, Rea LD (2006) Ontogeny of total body oxygen stores and aerobic dive potential in Steller sea lions (Eumetopias jubatus). J Comp Physiol B 176:535–545
Ridgway SH, Johnston DG (1966) Blood oxygen and ecology of porpoises of three genera. Science 151:456–457
Salathe EP, Chen C (1993) The role of myoglobin in retarding oxygen depletion in skeletal muscle. Math Biosci 116:1–20
Saunders DK, Fedde MR (1991) Physical conditioning: effect on the myoglobin concentration in skeletal and cardiac muscle of bar-headed geese. Comp Biochem Physiol A 100:349–352
Schreer JF, Kovacs KM, O’Hara Hines RJ (2001) Comparative diving patterns of pinnipeds and seabirds. Ecol Monogr 71(1):137–162
Stephenson R, Turner DL, Butler PJ (1989) The relationship between diving activity and oxygen storage capacity in the tufted duck (Aythya fuligula). J Exp Biol 141:265–275
Thorson PH (1993) Development of diving in the northern elephant seal. Ph.D. diss., University of California, Santa Cruz
Velten BP (2012) A comparative study of the locomotor muscle of extreme deep-diving-Cetaceans. M.S., University of North Carolina, Wilmington
Velten BP, Dillaman RM, Kinsey ST, McLellan WA, Pabst DA (2013) Novel locomotor muscle design in extreme deep-diving whales. J Exp Biol 216:1862–1871
Verrier D, Guinet C, Authier M, Tremblay Y, Shaffer S, Costa DP, Groscolas R, Arnould JPY (2011) The ontogeny of diving abilities in subantarctic fur seal pups: developmental trade-off in response to extreme fasting? Funct Ecol 25(4):818–828
Weber RE, Hemmingsen EA, Johansen K (1974) Functional and biochemical studies of penguin myoglobin. Comp Biochem Physiol B 49:197–214
Weise MJ, Costa DP (2007) Total body oxygen stores and physiological diving capacity of California sea lions as a function of sex and age. J Exp Biol 210:278–289
Wells RS (1991) Bringing up baby. Nat Hist 100(8):56–62
Westgate AJ, Read AJ, Berggren P, Koopman HN, Gaskin DE (1995) Diving behaviour of harbour porpoise, Phocoena phocoena. Can J Fish Aquat Sci 52:1064–1073
Williams TM, Noren SR, Glenn M (2011) Extreme physiological adaptations as predictors of climate-change sensitivity in the narwhal, Monodon monoceros. Mar Mamm Sci 27(2):334–349
Acknowledgments
Collection of samples was supported in part by funding from the John H. Prescott Marine Mammal and Rescue Assistance Grant through NOAA Fisheries. Analysis of samples was supported by NOAA Northwest Fisheries Science Center. We thank the volunteers and staff at the San Juan Marine Mammal Stranding Network and the Whale Museum, especially A. Traxler, for providing samples for this study. We also thank G. Ylitalo and L. Rhodes and their staff at the NOAA Northwest Fisheries Science Center for providing laboratory equipment and bench space for sample analysis. We thank D. Somo for assistance with sample analysis and M.L. Dolar for providing raw data from Dolar et al. (1999). Finally, we thank the laboratory group of T.M Williams for providing insightful comments on previous versions of this manuscript. Collection of samples from stranded harbor porpoises was authorized by the NOAA Northwest Regional Office (now the West Coast Regional Office). All experiments comply with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Noren, S.R., Noren, D.P. & Gaydos, J.K. Living in the fast lane: rapid development of the locomotor muscle in immature harbor porpoises (Phocoena phocoena). J Comp Physiol B 184, 1065–1076 (2014). https://doi.org/10.1007/s00360-014-0854-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0854-8