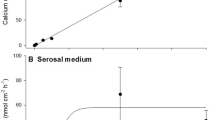
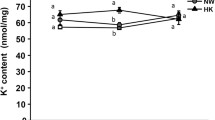
Abstract
Recent molecular evidence points towards a capacity for ammonia transport across the skin of adult rainbow trout. A series of in vivo and in vitro experiments were conducted to understand the role of cutaneous ammonia excretion (J amm) under control conditions and after 12-h pre-exposure to high environmental ammonia (HEA; 2 mmol/l NH4HCO3). Divided chamber experiments with bladder-catheterized, rectally ligated fish under light anesthesia were performed to separate cutaneous J amm from branchial, renal, and intestinal J amm. Under control conditions, cutaneous J amm accounted for 4.5 % of total J amm in vivo. In fish pre-exposed to HEA, plasma total ammonia concentration increased 20-fold to approximately 1,000 μmol/l, branchial J amm increased 1.5- to 2.7-fold, and urinary J amm increased about 7-fold. Urinary J amm still accounted for less than 2 % of total J amm. Cutaneous J amm increased 4-fold yet amounted to only 5.7 % of total J amm in these fish. Genes (Rhcg1, Rhcg2, Rhbg, NHE-2, v-type H+-ATPase) known to be involved in ammonia excretion at the gills of trout were all expressed at the mRNA level in the skin, but their expression did not increase with HEA pre-exposure. In vitro analyses using [14C] methylamine (MA), an ammonia analog which is transported by Rh proteins, demonstrated that MA permeability in isolated skin sections was higher in HEA pre-exposed fish than in control fish. The addition of basolateral ammonia (1,000 μmol/l) to this system abolished this increase in permeability, suggesting ammonia competition with MA for Rh-mediated transport across the skin of HEA pre-exposed trout; this did not occur in skin sections from control trout. Moreover, in vitro J amm by the skin of fish which had been pre-exposed to HEA was also higher than in control fish in the absence of basolateral ammonia, pointing towards a possible cutaneous ammonia loading in response to HEA. In vitro MA permeability was reduced upon the addition of amiloride (10−4 mol/l), but not phenamil (10−5 mol/l) suggesting a role for a Na/H-exchanger (NHE) in cutaneous ammonia transport, as has been previously described in the skin of larval fish. Overall, it appears that under control conditions and in response to HEA pre-exposure, the skin makes only a very minor contribution to total J amm, but the observed increases in cutaneous J amm in vivo and in cutaneous J amm and MA permeability in vitro demonstrate the capacity for ammonia transport in the skin of adult trout. It remains unclear if this capacity may become significant under certain environmental challenges or if it is merely a remnant of cutaneous transport capacity from early life stages in these fish.







Similar content being viewed by others
References
Chopin LK, Amey AP, Bennett MB (1998) A systemic secondary vessel system is present in the teleost fish Tandanus tandanus and absent in the elasmobranchs Carcharhinus melanopterus and Rhinobatos typus and in the dipnoan Neoceratodus forsteri. J Zool Lond 246:105–110
Cooper CA, Wilson JM, Wright PA (2013) Marine, freshwater and aerially acclimated mangrove rivulus (Kryptolebias marmoratus) use different strategies for cutaneous ammonia excretion. Am J Physiol Regul Integr Physiol 304:R599–R612
Frick NT, Wright PA (2002) Nitrogen metabolism and excretion in the mangrove killifish Rivulus marmoratus II. Significant ammonia volatilization in a teleost during air-exposure. J Exp Biol 205:91–100
Fromm PO (1968) Some quantitative aspects of ion regulation in teleosts. Comp Biochem Physiol 27:865–869
Fu C, Wilson JM, Rombough PJ, Brauner CJ (2010) Ions first: Na+ uptake shifts from the skin to the gills before O2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proc R Soc B 277:1553–1560
Glover CN, Bucking C, Wood CM (2011) Adaptations to in situ feeding: novel nutrient acquisition pathways in an ancient vertebrate. Proc R Soc B 278:3096–3101
Glover CN, Bucking C, Wood CM (2013) The skin of fish as a transport epithelium: a review. J Comp Physiol B 183:877–891
Hung CYC, Tsui KNT, Wilson JM, Nawata CM, Wood CM, Wright PA (2007) Rhesus glycoprotein gene expression in the mangrove killfish Kryptolebias marmoratus exposed to elevated environmental ammonia levels and air. J Exp Biol 210:2419–2429
Ishimatsu A, Iwama GK, Bentley TB, Heisler N (1992) Contribution of the secondary circulatory system to acid-base regulation during hypercapnia in rainbow trout (Oncorhynchus mykiss). J Exp Biol 170:43–56
Ito Y, Kobayashi S, Nakamura N, Miyagi H, Esaki M, Hoshijima K, Hirose S (2013) Close association of carbonic anhydrase (CA2a and CA15a), Na+/H+ exchanger (Nhe3b), and ammonia transporter Rhcg1 in zebrafish ionocytes responsible for Na+ uptake. Front Physiol 4:1–17
Kirsch R (1972) The kinetics of peripheral exchanges of water and electrolytes in the silver eel (Anguilla anguilla L.) in fresh water and in sea water. J Exp Biol 57:489–512
Kirsch R, Nonnotte G (1977) Cutaneous respiration in three freshwater teleosts. Respir Physiol 29:339–354
Kumai Y, Perry SF (2011) Ammonia excretion via Rhcg1 facilitates Na+ uptake in larval zebrafish, Danio rerio, in acidic water. Am J Physiol Regul Integr Comp Physiol 301:R1517–R1528
Liew JH, Sinha AK, Nawata CM, Blust R, Wood CM, De Boeck G (2013) Differential responses in ammonia excretion, sodium fluxes, and gill permeability explain different sensitivities to acute high environmental ammonia in three freshwater teleosts. Aquatic Tox 126:63–76
McDonald DG, Wood CM (1981) Branchial and renal acid and ion fluxes in the rainbow trout, Salmo gairdneri, at low environmental pH. J Exp Biol 93:101–118
McFarland WN (1959) A study of the effects of anesthetics on the behavior and physiology of fishes. Publ Inst Mar Sci Univ Tex 6:23–55
McKim JM, Schmieder PK, Carlson RW, Hunt EP (1987) Use of respiratory-cardiovascular responses of rainbow trout (Salmo gairdneri) in identifying acute toxicity syndromes in fish: part 1. Pentachlorophenol, 2,4-dinitrophenol, tricaine methanesulfonate and 1-octanol. Environ Toxicol Chem 6:295–312
Nakada T, Hoshijima K, Esaki M, Nagayoshi S, Kawakami K, Hirose S (2007) Localization of ammonia transporter Rhcg1 in mitochondrion-rich cells of yolk sac, gill, and kidney of zebrafish and its ionic strength-dependent expression. Am J Physiol Regul Integr Comp Physiol 293:R1743–R1753
Nawata CM, Wood CM (2008) The effects of CO2 and external buffering on ammonia excretion and Rhesus glycoprotein mRNA expression in rainbow trout. J Exp Biol 211:3226–3236
Nawata CM, Wood CM (2009) mRNA expression analysis of the physiological responses to ammonia infusion in rainbow trout. J Comp Physiol B 179:799–810
Nawata CM, Hung CYC, Tsui TKN, Wilson JM, Wright PA, Wood CM (2007) Ammonia excretion in rainbow trout (Oncorhynchus mykiss): evidence for Rh glycoprotein and H+-ATPase involvement. Physiol Genomics 31:463–474
Nawata CM, Hirose S, Nakada T, Wood CM, Kato A (2010a) Rh glycoprotein expression is modulated in pufferfish (Takifugu rubripes) during high environmental ammonia exposure. J Exp Biol 213:3150–3160
Nawata CM, Wood CM, O’Donnell MJ (2010b) Functional characterization of Rhesus glycoproteins from an ammoniotelic teleost, the rainbow trout, using oocyte expression and SIET analysis. J Exp Biol 213:1049–1059
Olson KR (1996) Secondary circulation in fish: anatomical organization and physiological significance. J Exp Zool 275:172–185
Perry SF, Malone S, Ewing D (1987) Hypercapnic acidosis in the rainbow trout (Salmo gairdneri) II. Renal ionic fluxes. Can J Zool 65:896–902
Rombough PJ (1999) The gill of fish larvae. Is it primarily a respiratory or an ionoregulatory structure? J Fish Biol 55:186–204
Sashaw J, Nawata M, Thompson S, Wood CM, Wright PA (2010) Rhesus glycoprotein and urea transporter genes in rainbow trout embryos are upregulated in response to alkaline water (pH 9.7) but not elevated water ammonia. Aquat Tox 96:308–313
Satchell GH (1991) Physiology and form of fish circulation. Cambridge University Press, Cambridge
Sayer MDJ, Davenport J (1987) The relative importance of the gills to ammonia and urea excretion in five seawater and one freshwater teleost species. J Fish Biol 31:561–570
Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, Handlogten ME, Verlander JW, Weiner ID (2006a) Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290:F397–F408
Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID (2006b) Changes in subcellular distribution of the ammonia transporter, Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290:F1443–F1452
Shih TH, Horng JL, Hwang PP, Lin LY (2008) Ammonia excretion by the skin of zebrafish (Danio rerio) larvae. Am J Physiol Cell Physiol 295:1625–1632
Shih TH, Horng JL, Liu ST, Hwang PP, Lin LY (2012) Rhcg1 and NHE3b are involved in ammonium-dependent sodium uptake by zebrafish larvae acclimated to low-sodium water. Am J Physiol Regul Integr Comp Physiol 302:R84–R93
Sinha AK, Liew HJ, Nawata CM, Blust R, Wood CM, De Boeck G (2013) Modulation of Rh glycoproteins, ammonia excretion and Na+ fluxes in three freshwater teleosts when exposed chronically to high environmental ammonia. J Exp Biol 216:2917–2930
Skov PV, Bennett MB (2003) The secondary vascular system of Actinopterygii: interspecific variations in origins and investment. Zoomorph 122:181–190
Skov PV, Bennett MB (2004) Structural basis for control of secondary vessels in the long-finned eel Anguilla reinhardtii. J Exp Biol 207:3339–3348
Smith HW (1929) The excretion of ammonia and urea by the gills of fish. J Biol Chem 81:727–742
Smith AA, Zimmer AM, Wood CM (2012) Branchial and extrabranchial ammonia excretion in goldfish (Carassius auratus) following thermally induced gill remodelling. Comp Biochem Physiol A162:185–192
Steffensen JF, Lomholt JP, Vogel WOP (1986) In vivo observations on a specialized microvasculature, the primary and secondary vessels in fishes. Acta Zool 67:193–200
Swift DJ, Lloyd R (1974) Changes in urine flow rate and haematocrit value of rainbow trout Salmo gairdneri (Richardson) exposed to hypoxia. J Fish Biol 6:379–387
Tsui TKN, Hung CYC, Nawata CM, Wilson JM, Wright PA, Wood CM (2009) Ammonia transport in cultured gill epithelium of freshwater rainbow trout: the importance of Rhesus glycoproteins and the presence of an apical Na+/NH4 + exchange complex. J Exp Biol 212:878–892
Verdouw H, van Echteld CJA, Dekkers EMJ (1978) Ammonia determination based on indophenols formation with sodium salicylate. Water Res 12:399–402
Vogel WOP, Claviez M (1981) Vascular specialization in fish, but no evidence for lymphatics. Z Naturforsch 36c:490–492
Weihrauch D, Wilkie MP, Walsh PJ (2009) Ammonia and urea transporters in gills of fish and aquatic crustaceans. J Exp Biol 212:1716–1730
Wells PR, Pinder AW (1996) The respiratory development of Atlantic salmon II. Partitioning of oxygen uptake among gills, yolk sac and body surfaces. J Exp Biol 199:2737–2744
Wilson RW, Wright PM, Munger S, Wood CM (1994) Ammonia excretion in freshwater rainbow trout (Oncorhynchus mykiss) and the importance of gill boundary layer acidification: lack of evidence for Na+/NH4 + exchange. J Exp Biol 191:37–58
Wood CM (1988) Acid-base and ionic exchanges at gills and kidney after exhaustive exercise in the rainbow trout. J Exp Biol 136:461–481
Wood CM, Nawata CM (2011) Nose-to-nose comparison of physiological and molecular responses of trout to high environmental ammonia in seawater versus freshwater. J Exp Biol 214:3557–3569
Wood CM, Patrick ML (1994) Methods for assessing kidney and urinary bladder function in fish. In: Hochachka PW, Mommsen TP (eds) Biochemistry and Molecular Biology of Fishes, vol 3. pp 127–143
Wood CM, Gilmour KM, Part P (1998) Passive and active transport properties of a gill model, the cultured branchial epithelium of the freshwater rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol 119A:87–96
Wood CM, Milligan LM, Walsh PJ (1999) Renal responses of trout to chronic respiratory and metabolic acidosis and metabolic alkalosis. Am J Physiol 277:R482–R492
Wright PA, Wood CM (2009) A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins. J Exp Biol 212:2303–2312
Wu SC, Horng JL, Liu ST, Hwang PP, Wen ZH, Lin CS, Lin LY (2010) Ammonium-dependent sodium uptake in mitochondrion-rich cells of medaka (Oryzias latipes) larvae. Am J Physiol 298:C237–C250
Zimmer AM, Nawata CM, Wood CM (2010) Physiological and molecular analysis of the interactive effects of feeding and high environmental ammonia on branchial ammonia excretion and Na+ uptake in freshwater rainbow trout. J Comp Physiol B 180:1191–1204
Acknowledgments
Special thanks to Ryan Belowitz for assistance in skin surface area measurements and analyses, and to Jeff Richards and Eric Clelland for arranging access to [14C]methylamine. This research was supported by NSERC (Natural Sciences and Engineering Research Council of Canada) Discovery Grants to CJB and CMW, who is also supported by the Canada Research Chair Program. AZ was supported by an Ontario Graduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Zimmer, A.M., Brauner, C.J. & Wood, C.M. Ammonia transport across the skin of adult rainbow trout (Oncorhynchus mykiss) exposed to high environmental ammonia (HEA). J Comp Physiol B 184, 77–90 (2014). https://doi.org/10.1007/s00360-013-0784-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-013-0784-x