Abstract
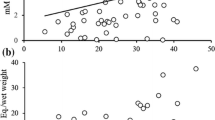
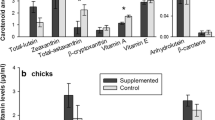
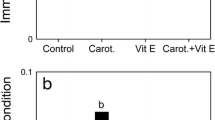
Carotenoids are considered a limited resource for animals because they are not synthesised by the body. Birds use carotenoids, mainly xanthophylls, for physiological functions, such as anti-oxidant activity, and for colour expression; hence, they need to shunt carotenoids between competitive demands. Recent studies suggest that the anti-oxidant role of xanthophylls might not be as important as previously thought and that at high concentrations they may, in fact, acquire pro-oxidant properties. In this work, we studied the effects of a moderate xanthophyll supplementation (115 mg of carotenoids/kg diet/day; 4 weeks) on serum carotenoids, serum concentration of reactive oxygen metabolites (ROMs), serum anti-oxidant capacity (OXY), the degree of oxidative stress (OS; ROMs/OXY × 1,000), body mass, and skin colour, in rehabilitated captive adult Eurasian kestrels (Falco tinnunculus). The supplementation caused increased levels of serum carotenoids (∼90%), ROMs (∼82%), OS (∼115%) and an immediate loss of body mass (∼6.2%), but it did not affect OXY and tarsi skin hue. The red (∼16%) and yellow (∼15%) colorimetric components were increased after the first week of supplementation and the effect persisted during the rest of the experiment. Two months after the end of supplementation, serum carotenoids, OS and ROMs returned to baseline levels, however the body mass did not. Our findings suggest that, above a certain physiological threshold, carotenoids can cause detrimental effects. This is relevant for the trade-off between expression of sexual signals and the costs of maintaining/producing them.



Similar content being viewed by others
References
Alonso-Alvarez C, Bertrand S, Devevey G, Gaillard M, Prost J, Faivre B, Sorci G (2004) An experimental test of the dose–dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am Nat 164:651–659
Bertrand S, Alonso-Alvarez C, Devevey G, Faivre B, Prost J, Sorci G (2006) Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia 147:576–584
Blas J, Pérez-Rodríguez L, Bortolotti GR, Viñuela J, Marchant TA (2006) Testosterone increases bioavailability of carotenoids: insights into the honesty of sexual signaling. Proc Natl Acad Sci USA 103:18633–18637
Blount JD (2004) Carotenoids and life-history evolution in animals. Arch Biochem Biophys 430:10–15
Blount JD, Møller AP, Houston DC (2001) Antioxidants, showy males and sperm quality. Ecol Lett 4:393–396
Blount JD, Metcalfe NB, Birkhead TR, Surai PF (2003) Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300:125–127
Brush AH (1990) Metabolism of carotenoid pigments in birds. FASEB J 4:2969–2977
Casagrande S, Csermely D, Pini E, Bertacche V, Tagliavini J (2006) Skin carotenoid concentration correlates with male hunting skill and territory quality in the kestrel (Falco tinnunculus). J Avian Biol 37:190–196
Casagrande S, Costantini D, Fanfani A, Tagliavini J, Dell’Omo G (2007) Patterns of serum carotenoid accumulation and skin color variation in nestling kestrels in relation to breeding conditions and different terms of carotenoid supplementation. J Comp Physiol B 177:237–245
Costantini D, Casagrande S, Di Lieto G, Fanfani A, Dell’Omo G (2005a) Consistent differences in feeding habits between neighbouring breeding kestrels. Behaviour 142:1409–1421
Costantini D, Dell’Omo G, Casagrande S, Fabiani A, Carosi M, Bertacche V, Marquez C, Snell H, Snell H, Tapia W, Gentile G (2005b) Inter-population variation of carotenoids in Galápagos land iguanas (Conolophus subcristatus). Comp Biochem Physiol B 142:239–244
Costantini D, Dell’Omo G (2006a) Effects of T-cell-mediated immune response on avian oxidative stress. Comp Biochem Physiol A 145:137–142
Costantini D, Dell’Omo G (2006b) Environmental and genetic components of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J Comp Physiol B 176:575–579
Costantini D, Casagrande S, De Filippis S, Brambilla G, Fanfani A, Tagliavini J, Dell’Omo G (2006) Correlates of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J Comp Physiol B 176:329–337
Costantini D, Fanfani A, Dell’Omo G (2007) Carotenoid availability does not limit the capability of nestling kestrels (Falco tinnunculus) to cope with oxidative stress. J Exp Biol 210:1238–1244
Czeczuga B (1978) Carotenoids in the skin of certain species of birds. Comp Biochem Physiol B 6:107–109
Czeczuga B (1979) Carotenoids in some parts of certain species of lizards. Comp Biochem Physiol B 65:755–757
Dijkstra C (1988) Reproductive tactics in the kestrel Falco tinnunculus. Ph.D. thesis, University of Groningen
Dijkstra C, Daan S, Meijer T, Cavé A.J, Foppen RPB (1988) Daily and seasonal variations in body mass of the kestrel in relation to food availability and reproduction. Ardea 76:127–140
Dotan Y, Lichtenberg D, Pinchuck I (2004) Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Progr Lip Res 43:200–227
El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, George Truscott T, Young AJ (2004) Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys 430:37–48
Faivre B, Grégoire A, Préault M, Cézilly F, Sorci G (2003) Immune activation rapidly mirrored in a secondary sexual trait. Science 300:103
Goodwin TW (1984) The biochemistry of carotenoids. Animals, vol II. Chapman and Hall, London
Grether GF, Hudon J, Millie DF (1999) Carotenoid limitation of sexual coloration along an environmental gradient in guppies. Proc R Soc Lond B 266:1317–1322
Gruszecki WI (1999) Carotenoids in membranes. In: Frank HA, Young AJ, Britton G, Cogdell RJ (eds) The photochemistry of carotenoids. Kluwer, Dordrecht, pp 363–379
Handelman GJ, van Kuijk FJ, Chatterjee A, Krinsky NI (1991) Characterization of products formed during the autoxidation of β-carotene. Free Rad Biol Med 10:427–437
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Hayes JP, Shonkwiler JS (1996) Analyzing mass-independent data. Physiol Zool 69:974–980
Hõrak P, Zilmer M, Saks L, Ots I, Karu U, Zilmer K (2006) Antioxidant protection, carotenoids, and the costs of immune challenge in greenfinches. J Exp Biol 209: 4329–4338
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lozano GA (1994) Carotenoids, parasites, and sexual selection. Oikos 70:309–311
McGraw KJ, Adkins-Regan E, Parker RS (2005) Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften 92:375–380
McGraw KJ, Correa SM, Adkins-Regan E (2006) Testosterone upregulates lipoprotein status to control sexual attractiveness in a colorful songbird. Behav Ecol Sociobiol 60:117–122
Møller AP, Biard C, Blount JD, Houston DC, Ninni P, Saino N, Surai PF (2000) Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult Biol Rev 11:137–159
Negro JJ, Bortolotti GR, Tella JL, Fernie KJ, Bird DM (1998) Regulation of integumentary colour and plasma carotenoids in American kestrel consistent with sexual selection theory. Funct Ecol 12:307–312
Negro JJ, Tella JL, Blanco G, Forero MG, Garrido-Fernández J (2000) Diet explains interpopulation variation of plasma carotenoids and skin pigmentation in nestling white storks. Physiol Biochem Zool 73:97–101
Negro JJ, Figuerola J, Garrido J, Green AJ (2001) Fat stores in birds: an overlooked sink for carotenoid pigments? Funct Ecol 15:297–303
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
Owens IPF, Short RV (1995) Hormonal basis of sexual dimorphism in birds: implications for new theories of sexual coloration. Trends Ecol Evol 10:44–47
Palozza P (1998) Prooxidant actions of carotenoids in biological systems. Nutr Rev 56:257–265
Partali V, Liaaen-Jensen S, Slagsvold T, Lifjeld JT (1987) Carotenoids in food chain studies II. The food chain of Parus spp. monitored by carotenoid analysis. Comp Biochem Physiol B 87:885–888
Peters A, Denk AG, Delhey K, Kempenaers B (2004) Carotenoid-based bill colour as an indicator of immunocompetence and sperm performance in male mallards. J Evol Biol 17:1111–1120
Roff DA (1992) The evolution of life histories. Chapman and Hall, New York
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B 266:1–12
Siems WG, Sommerburg O, Hurst JS, van Kuijk FJGM (2000) Carotenoid oxidative degradation products inhibit Na+-K+-ATPase. Free Rad Res 33:427–435
Siems W, Wiswedel I, Salerno C, Crifò C, Augustin W, Schild L, Langhans C-D, Sommerburg O (2005) β-carotene breakdown products may impair mitochondrial functions-potential side effects of high-dose β-carotene supplementation. J Nutr Biochem 16:358–397
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Surai P (2002) Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham
Village A (1990) The kestrel. T & AD Poyser, London
Tummeleht L, Mägi M, Kilgas P, Mänd R, Hõrak P (2006) Antioxidant protection and plasma carotenoids of incubating great tits (Parus major L.) in relation to health state and breeding conditions. Comp Biochem Physiol C 144: 166–172
Wefers H, Sies H (1988) The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem 174:353–357
Woodall AA, Britton G, Jackson MJ (1996) Dietary supplementation with carotenoids: effects on α-tocopherol levels and susceptibility of tissues to oxidative stress. Br J Nutr 76:307–317
Young AJ, Lowe GM (2001) Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biop 385:20–27
Zahavi A, Zahavi A (1997) The handicap principle: a missing piece of Darwin’s puzzle. Oxford University Press, Oxford
Acknowledgments
We are grateful to the rehabilitation centres of the Lega Italiana Protezione Uccelli (LIPU) sections of Latina and Roma, Circeo National Park, Forestry Service of Pescara, Bosco San Silvestro (Caserta), Vico Natural Reserve (Caprarola, VT) and WWF Astroni for providing the birds. We thank two anonymous reviewers for valuable comments that improved the manuscript; Stefania Casagrande for valuable comments that improved an earlier draft; Eugenio Sodo from Kemin Food that kindly provided the carotenoids used for the experiment; Nadia Macciocchi, Fernando and Serena Costantini for help with the animal care; the scientific association Ornis italica for supporting this research; Gianfranco Brambilla and Maurizio Fiori for valuable suggestions. David Costantini was supported by a Ph.D. fellowship from the University La Sapienza.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Costantini, D., Coluzza, C., Fanfani, A. et al. Effects of carotenoid supplementation on colour expression, oxidative stress and body mass in rehabilitated captive adult kestrels (Falco tinnunculus). J Comp Physiol B 177, 723–731 (2007). https://doi.org/10.1007/s00360-007-0169-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0169-0