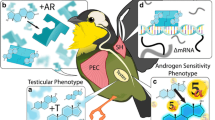
Abstract
A hallmark of sexual selection is the evolution of elaborate male sexual signals. Yet, how the physiology of an animal changes to support a new or modified signal is a question that has remained largely unanswered. Androgens are important in regulating male reproductive behavior, therefore, selection for particular signals may drive the evolution of increased androgenic sensitivity in the neuro-motor systems underlying their production. Studies of the neuroendocrine mechanisms of anuran sexual signaling provide evidence to support this idea. Here, we highlight two such cases: first, a large body of work in Xenopus frogs demonstrates that sexually dimorphic androgen receptor (AR) expression in the laryngeal nerves and muscles underlies sexually dimorphic vocal behavior, and second, our own work showing that the recent evolution of a hind limb signal (known as the “foot flag”) in Staurois parvus is accompanied by a dramatic increase in androgenic sensitivity of the thigh muscles that control limb movement. Together, these examples illustrate that the evolutionary modification or gain of a sexual signal is linked with a novel pattern of AR expression in the tissues that support it. We suggest that such co-evolution of AR expression and sex-specific or species-specific signaling behavior exists across vertebrates.


Similar content being viewed by others
References
Adkins-Regan E (2005) Hormones and animal social behavior. Monographs in behavior and ecology. Princeton University Press, Princeton
Amézquita A, Hödl W (2004) How, when, and where to perform visual displays? The case of the Amazonian frog Hyla parviceps. Herpetologica 60:20–29
Ball GF (2003) Neuroendocrine basis of seasonal changes in vocal behavior among songbirds. In: Hauser M, Konishi M (eds) The design of animal communication. Cambridge, pp 213–253
Barkan CL, Zornik E, Kelley DB (2017) Evolution of vocal patterns: tuning hindbrain circuits during species divergence. J Exp Biol 220:856–867. doi:10.1242/jeb.146845
Bass AH, Remage-Healey L (2008) Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav 53:659–672. doi:10.1016/j.yhbeh.2007.12.010
Boistel R, Sueur J (2002) Female calls among amphibian anura: an exceptional sound behavior? Bioacoustics 13:80–81
Brantley RK, Marchaterre MA, Bass AH (1993a) Androgen effects on vocal muscle structure in a teleost fish with inter- and intra-sexual dimorphism. J Morphol 216:305–318. doi: 10.1002/jmor.1052160306
Brantley RK, Wingfield JC, Bass AH (1993b) Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm Behav 27:332–347. doi: 10.1006/hbeh.1993.1025
Breedlove MS, Arnold AP (1981) Sexually dimorphic motor nucleus in the rat lumbar spinal cord: Response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res 225:297–307. doi: 10.1016/0006-8993(81)90837-4
Breedlove SM, Arnold AP (1983) Sex differences in the pattern of steroid accumulation by motoneurons of the rat lumbar spinal cord. J Comp Neurol 215:211–216. doi:10.1002/cne.902150208
Bro-Jørgensen J (2010) Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol Evol (Amst) 25:292–300. doi:10.1016/j.tree.2009.11.003
Catz DS, Fischer LM, Moschella MC, Tobias ML (1992) Sexually dimorphic expression of a laryngeal-specific, androgen-regulated myosin heavy chain gene during Xenopus laevis development. Dev Biol 154:366–376. doi:10.1016/0012-1606(92)90075-R
Catz DS, Fischer LM, Kelley DB (1995) Androgen regulation of a laryngeal-specific myosin heavy chain mRNA isoform whose expression is sexually differentiated. Dev Biol 171:448–457. doi:10.1006/dbio.1995.1295
Clarke JA, Chatterjee S, Li Z, Riede T, Agnolin F, Goller F, Isasi MP, Martinioni DR, Mussel FJ, Novas FE (2016) Fossil evidence of the avian vocal organ from the Mesozoic. Nature 538:502–505
Crews D, Moore MC (1986) Evolution of mechanisms controlling mating behavior. Science 231:121–125
Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, Molinaro M, Rosenthal N, Musaro A (2005) Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol 168:193–199
Emerson SB, Boyd SK (1999) Mating vocalizations of female frogs: control and evolutionary mechanisms. Brain Behav Evol 53:187–197
Endler JA (1993) Some general comments on the evolution and design of animal communication systems. Philos Trans R Soc B 340:215–225. doi:10.1098/rstb.1993.0060
Endler JA (2015) Signals, signal conditions, and the direction of evolution. Am Nat 139:S125–S153. doi:10.1086/285308
Feng NY, Katz A, Day LB, Barske J, Schlinger BA (2010) Limb muscles are androgen targets in an acrobatic tropical bird. Endocrinology 151:1042–1049. doi:10.1210/en.2009-0901
Fischer LM, Kelley DB (1991) Androgen receptor expression and sexual differentiation of effectors for courtship song in Xenopus laevis. Sem Neurosci 3:469–480. doi:10.1016/1044-5765(91)90056-T
Fusani L, Day LB, Canoine V, Reinemann D, Hernandez E, Schlinger BA (2007) Androgen and the elaborate courtship behavior of a tropical lekking bird. Horm Behav 51:62–68. doi:10.1016/j.yhbeh.2006.08.005
Fuxjager MJ, Schlinger BA (2015) Perspectives on the evolution of animal dancing: a case study of manakins. Curr Opin Behav Sci 6:7–12. doi:10.1016/j.cobeha.2015.06.007
Fuxjager MJ, Barske J, Du S, Day LB, Schlinger BA (2012a) Androgens regulate gene expression in avian skeletal muscles. PLoS One. doi:10.1371/journal.pone.0051482
Fuxjager MJ, Schultz JD, Barske J, Feng NY, Fusani L, Mirzatoni A, Day LB, Hau M, Schlinger BA (2012b) Spinal motor andsensory neurons are androgen targets in an acrobatic bird. Endocrinology 153:3780–3791. doi:10.1210/en.2012-1313
Fuxjager MJ, Longpre KM, Chew JG, Fusani L, Schlinger BA (2013) Peripheral androgen receptors sustain the acrobatics and fine motor skill of elaborate male courtship. Endocrinology 154:3168–3177. doi:10.1210/en.2013-1302
Fuxjager MJ, Heston JB, Schlinger BA (2014) Peripheral androgen action helps modulate vocal production in a suboscine passerine. Auk 131:327–334. doi:10.1642/AUK-13-252.1
Fuxjager MJ, Lee J-H, Chan T-M, Bahn JH, Chew JG, Xiao X, Schlinger BA (2016) Research resource: hormones, genes, and athleticism: effect of androgens on the avian muscular transcriptome. Mol Endocrinol 30:254–271. doi:10.1210/me.2015-1270
Fuxjager MJ, Miles MC, Goller F, Petersen J, Yancey J (2017) Androgens enhance male acrobatic courtship by enhancing muscle speed and easing the severity of its trade-off with force. Endocrinology. doi:10.1210/en.2017-00599
Grafe TU, Preininger D, Sztatecsny M, Kasah R, Dehling JM, Proksch S, Hödl W (2012) Multimodal communication in a noisy environment: a case study of the Bornean rock frog Staurois parvus. PLoS One 7:e37965. doi:10.1371/journal.pone.0037965
Guerra MA, Ryan MJ, Cannatella DC (2014) Ontogeny of sexual dimorphism in the larynx of the túngara frog, Physalaemus pustulosus. Copeia 2014:123–129
Harrington AW, Ginty DD (2013) Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci 14:177–187
Hau M (2007) Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29:133–144. doi:10.1002/bies.20524
Hödl W, Amézquita A (2001) Visual signaling in anuran amphibians. In: Ryan MJ (ed) Anuran communication. Smithsonian, Washington DC
Holmes MM, Wade J (2005) Testosterone regulates androgen receptor immunoreactivity in the copulatory, but not courtship, neuromuscular system in adult male green anoles. J Neuroendocrinol 17:560–569
Holmes MM, Bartrem CL, Wade J (2007) Androgen dependent seasonal changes in muscle fiber type in the dewlap neuromuscular system of green anoles. Physiol Behav 91:601–608. doi:10.1016/j.physbeh.2007.03.022
Hoskin CJ, Higgie M (2010) Speciation via species interactions: the divergence of mating traits within species. Ecol Lett 13:409–420. doi:10.1111/j.1461-0248.2010.01448.x
Jordan CL, Breedlove SM, Arnold AP (1991) Ontogeny of steroid accumulation in spinal lumbar motoneurons of the rat: implications for androgen’s site of action during synapse elimination. J Comp Neurol 313:441–448. doi:10.1002/cne.903130304
Kaspar BK (2003) Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301:839–842
Kelley DB (1986) Neuroeffectors for vocalization in Xenopus laevis: hormonal regulation of sexual dimorphism. Dev Neurobiol 17:231–248. doi:10.1002/neu.480170307
Kelley DB (2002) Hormonal regulation of motor output in amphibians: Xenopus laevis vocalizations as a model system. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin R (eds) Hormones, brain and behavior, vol 2. Academic, Amsterdam, pp 445–468
Kelley D, Sassoon D, Segil N, Scudder M (1989) Development and hormone regulation of androgen receptor levels in the sexually dimorphic larynx of Xenopus laevis. Dev Biol 131:111–118. doi:10.1016/S0012-1606(89)80042-9
Lee JSF, Bass AH (2005) Differential effects of 11-ketotestosterone on dimorphic traits in a teleost with alternative male reproductive morphs. Horm Behav 47:523–531. doi:10.1016/j.yhbeh.2005.01.003
Leininger EC, Kelley DB (2015) Evolution of courtship songs in Xenopus: Vocal pattern generation and sound production. Cytogenet Genome Res 145:302–314. doi:10.1159/000433483
Leininger EC, Kitayama K, Kelley DB (2015) Species-specific loss of sexual dimorphism in vocal effectors accompanies vocal simplification in African clawed frogs (Xenopus). J Exp Biol 218:849–857. doi:10.1242/jeb.115048
Mangiamele LA, Fuxjager MJ, Schuppe ER, Taylor RS, Hödl W, Preininger D (2016) Increased androgenic sensitivity in the hind limb muscular system marks the evolution of a derived gestural display. Proc Natl Acad Sci USA 113:5664–5669. doi:10.1073/pnas.1603329113
McClelland BE, Wilczynski W (1989) Sexually dimorphic laryngeal morphology in Rana pipiens. J Morphol 201:293–299. doi:10.1002/jmor.1052010308
McClelland B, Wilczynski W, Rand AS (1997) Sexual dimorphism and species differences in the neurophysiology and morphology of the acoustic communication system of two neotropical hylids. J Comp Physiol A 180:451–462. doi:10.1007/s003590050062
Nasipak B, Kelley DB (2012) Developing laryngeal muscle of Xenopus laevis as a model system: androgen-driven myogenesis controls fiber type transformation. Dev Neurobiol 72:664–675. doi:10.1002/dneu.20983
Nelson RJ (2000) An introduction to behavioral endocrinology, 2nd edn. Sinauer Associates, Inc., Sunderland
Perez J, Kelley DB (1996) Trophic effects of androgen: receptor expression and the survival of laryngeal motor neurons after axotomy. J Neurosci 16:6625–6633
Perez J, Cohen MA, Kelley DB (1996) Androgen receptor mRNA expression in Xenopus laevis CNS: sexual dimorphism and regulation in laryngeal motor nucleus. J Neurobiol 30:556–568
Podos J (2001) Correlated evolution of morphology and vocal signal structure in Darwin’s finches. Nature 409:185
Preininger D, Boeckle M, Hödl W (2009) Communication in noisy environments II: visual signaling behavior of male foot-flagging frogs, Staurois latopalmatus. Herpetologica 65:166–173. doi:10.1655/08-037R.1
Preininger D, Weissenbacher A, Wampula T, Hödl W (2012) The conservation breeding of two foot-flagging frog species from Borneo, Staurois parvus and Staurois guttatus. Amphib Reptile Conserv 5:45–56
Preininger D, Boeckle M, Sztatecsny M, Hödl W (2013) Divergent receiver responses to components of multimodal signals in two foot-flagging frog species. PLoS One. doi:10.1371/journal.pone.0055367
Preininger D, Handschuh S, Boeckle M, Sztatecsny M, Hödl W (2016) Comparison of female and male vocalisation and larynx morphology in the size dimorphic foot-flagging frog species, Staurois guttatus. Herpetol J 26:187–197
Přikryl T, Aerts P, Havelková P, Herrel A, Rocek Z (2009) Pelvic and thigh musculature in frogs (Anura) and origin of anuran jumping locomotion. J Anat 214:100–139
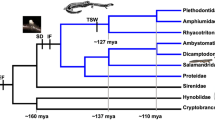
Pyron RA, Wiens JJ (2011) A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol 61:543–583
Rand MN, Breedlove SM (1992) Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol 23:17–30
Rand MN, Breedlove SM (1995) Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci 15:4408–4416
Remage-Healey L, Bass AH (2004) Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci 24:5892–5900. doi:10.1523/JNEUROSCI.1220-04.2004
Remage-Healey L, Bass AH (2006) From social behavior to neural circuitry: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm Behav 50:432–441. doi:10.1016/j.yhbeh.2006.05.007
Ryan MJ, Rand AS (1993) Species recognition and sexual selection as a unitary problem in animal communication. Evol Int J org Evol 47:647–657. doi:10.2307/2410076
Sartor JJ, Balthazart J, Ball GF (2005) Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria). Horm Behav 47:467–476. doi:10.1016/j.yhbeh.2004.12.004
Sassoon D, Kelley DB (1986) The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am J Anat 177:457–472. doi:10.1002/aja.1001770404
Sassoon DA, Gray GE, Kelley DB (1987) Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J Neurosci 7:3198–3206
Schlinger BA, Barske J, Day L, Fuasi L, Fuxjager MJ (2013) Hormones and the neuromuscular control of courtship in the golden-collared manakin (Manacus vitellinus). Front Neuroendocrinol 34:143–156. doi:10.1016/j.yfrne.2013.04.001
Searcy WA, Nowicki S (2005) The evolution of animal communication. Princeton University Press, Princeton
Segil N, Silverman L, Kelley DB (1987) Androgen-binding levels in a sexually dimorphic muscle of Xenopus laevis. Gen Comp Endocrinol 66:95–101. doi:10.1016/0016-6480(87)90354-6
Tobias ML, Kelley DB (1987) Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J Neurosci 7:3191–3197
Tobias ML, Marin ML, Kelley DB (1991) Development of functional sex differences in the larynx of Xenopus laevis. Dev Biol 147:251–259. doi:10.1016/S0012-1606(05)80022-3
Tobias ML, Marin ML, Kelley DB (1993) The roles of sex, innervation, and androgen in laryngeal muscle of Xenopus laevis. J Neurosci 13:324–333
Tobias ML, Evans BJ, Kelley DB (2011) Evolution of advertisement calls in African clawed frogs. Behaviour 148:519–549. doi:10.1163/000579511X569435
Veney SL, Wade J (2004) Steroid receptors in the adult zebra finch syrinx: a sex difference in androgen receptor mRNA, minimal expression of estrogen receptor α and aromatase. Gen Comp Endocrinol 136:192–199. doi:10.1016/j.ygcen.2003.12.017
Verhovshek T, Sengelaub DR (2013) Androgen action at the target musculature regulates brain-derived neurotrophic factor protein in the spinal nucleus of the bulbocavernosus. Dev Neurobiol 73:587–598
Verhovshek T, Buckley KE, Sergent MA, Sengelaub DR (2009) Testosterone metabolites differentially maintain adult morphology in a sexually dimorphic neuromuscular system. Dev Neurobiol 70:206–221
Verhovshek T, Cai Y, Osborne NC, Sengelaub SR (2010) Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology 151:253–261
Wade J, Buhlman L (2000) Lateralization and effects of adult androgen in a sexually dimorphic neuromuscular system controlling song in zebra finches. J Comp Neurol 426:154–164
Wetzel DM, Kelley DB (1983) Androgen and gonadotropin effects on male mate calls in South African clawed frogs,Xenopus laevis. Horm Behav 17:388–404
Wiens JJ (2001) Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol Evol 16:517–523
Wiley RH, Richards DG (1978) Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav Ecol Sociobiol 3:69–94. doi:10.1007/BF00300047
Wingfield JC, Hegner RE, Dufty AM Jr (1990) The” challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846. doi:10.1086/285134
Zornik E, Kelley DB (2008) Regulation of respiratory and vocal motor pools in the isolated brain of Xenopus laevis. J Neurosci 28:612–621. doi:10.1523/JNEUROSCI.4754-07.2008
Zornik E, Kelley DB (2011) A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front Neuroendocrinol 32:353–366. doi:10.1016/j.yfrne.2010.12.006
Zornik E, Kelley DB (2017) Hormones and vocal systems: insights from Xenopus. In: Pfaff D, Joëls M (eds) Hormones, brain, and behavior, vol 2, 3rd edn. Elsevier, Amsterdam, pp 117–144
Zornik E, Yamaguchi A (2011) Vocal pathway degradation in gonadectomized Xenopus laevis adults. J Neurophysiol 105:601–614
Acknowledgements
Supported by NSF IOS-1655574 to L.A.M and M.J.F. All animal experiments that are the authors’ original work have been approved by the IACUC of Smith College and Wake Forest University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mangiamele, L.A., Fuxjager, M.J. Insight into the neuroendocrine basis of signal evolution: a case study in foot-flagging frogs. J Comp Physiol A 204, 61–70 (2018). https://doi.org/10.1007/s00359-017-1218-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1218-0