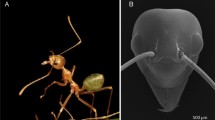
Abstract
Adult males of the insect order Strepsiptera are characterized by an unusual visual system that may use design principles from compound as well as simple eyes. The lenses of this eye are unusually large and focus images onto extended retinae. The light-gathering ability of the lens is sufficient to resolve multiple points of an image in each optical unit. We regard each unit as an independent image-forming eye that contributes an inverted partial image. Each partial image is re-inverted by optic chiasmata between the retinae and the lamina, where the complete image could be assembled from the neighboring units. The lamina, medulla and lobula are present, but their organization into cartridges is not clearly discernable. Fluorescent fills, whole-tissue stains, and synaptotagmin immunohistochemistry show that the optic neuropils nevertheless are densely packed, and that several parallel channels within the medulla underlie each of the lenses. The size and shape of the rhabdoms, as well as a relatively slow flicker-fusion frequency could suggest that these eyes evolved through a nocturnal life stage.









Similar content being viewed by others
Abbreviations
- O :
-
object size
- U :
-
object distance
- I :
-
image size
- f :
-
focal length
- A :
-
lens aperture
- D :
-
lens diameter
- Δφ:
-
interommatidial angle
- S :
-
light sensitivity of optical system
References
Autrum H (1958) Electrophysiological analysis of the visual systems in insects. Exp Cell Res [Suppl] 5:426–439
Bodian D (1937) A new method for staining nerve fibers and nerve endings in mounted paraffin sections. Anat Rec 69:153–162
Bruno MS, Barnes SN, Goldsmith TH (1977) Visual pigment and visual cycle of lobster, Homarus. J Comp Physiol A 120:123–142
Brunschwig AS, Salt AN (1997) Fixation-induced shrinkage of Reissner's membrane and its potential influence on the assessment of endolymph volume. Hearing Research 114:62–68
Buschbeck EK, Strausfeld NJ (1997) The relevance of neural architecture to visual performance: phylogenetic conservation and variation in dipteran visual systems. J Comp Neurol 383:282–304
Buschbeck EK, Ehmer B, Hoy RR (1999) Chunk versus point sampling: visual imaging in a small insect. Science 286:1178–1180
Cajal SR, Sánchez D (1915) Contribución al conocimiento de los centros nerviosos de los insectos. Parte I. Retina y centros ópticos. Trab Lab Invest Biol Univ Madrid 13:1-165
Campan R, Gallo A, Queinnec Y (1965) Détermination électrorétinographique de la fréquence critique fusionnement visuel: étude comparative portant sur les yeux composés de dix-sept espèces d'insectes. C R Soc Biol Paris 159:2521–2526
Davenport HA, Windle WF, Buch RH (1934) Block staining of nervous tissue. IV. Embryos. Stain Technol 29:165–173
DeYoe EA, Van Essen DC (1988) Concurrent processing streams in monkey visual cortex. Trends Neurosci 11:219–226
Felsenstein J (1978) Cases in which parsimony or compatibility methods will be positively misleading. Syst Zool 27:401–410
Fischbach K-F, Dittrich PM (1989) The optic lobes of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res 258:441–475
Fordyce D, Cronin TW (1989) Comparison of fossilized schizochroal compound eyes of phacopid trilobites with eyes of modern marine crustaceans and other arthropods. J Crust Biol 9:554–569
Fordyce D, Cronin TW (1993) Trilobite vision: a comparison of schizochroal and holochroal eyes with the compound eyes of modern arthropods. Paleobiology 19:288–303
Fouchard R, Carricaburu P (1972) Analyse de l'electroretinogramme de l'insecte. Vision Res 12:1-15
Fry MA, Tarsitano M, Dickinson MH (2003) Odor localization requires visual feedback during free flight in Drosophila melanogaster. J Exp Biol 206:843–855
Goldsmith TH (1960) The nature of the retinal action potential, and the spectral sensitivities of ultraviolet and green receptor systems of the compound eye of the worker honeybee. J Gen Physiol 43:775–800
Gregory GE (1980) The Bodian protargol technique. In: Strausfeld NJ, Miller TA (eds) Neuroanatomical techniques. Springer, Berlin Heidelberg New York, pp 75–95
Hardie RC (1979) Electrophysiological analysis of fly retina. I. comparative properties of R1–6 and R7 and 8. J Comp Physiol 129:19–33
Heisenberg M (1971) Separation of receptor and lamina potentials in the electroretinogram of normal and mutant Drosophila. J Exp Biol 55:85–100
Horváth G, Clarkson ENK, Pix W (1997) Survey of modern counterparts of schizochroal trilobite eyes: structural and functional similarities and differences. Hist Biol 12:229–263
Huelsenbeck JP (1998) Systematic bias in phylogenetic analysis: is the Strepsiptera problem solved? Syst Biol 47:519–537
Huelsenbeck JP (2001) A Bayesian perspective on the Strepsiptera problem. Tijdschr Entomol 144:165–178
Huelsenbeck JP, Hillis DM (1993) Success of phylogenetic methods in the four-taxon case. Syst Biol 42:247–264
Kathirithamby J (1989) Review of the order Strepsiptera. Syst Entomol 14:41–92
Kathirithamby J (1992) Strepsiptera of Panama and Mesoamerica. In: Quintero D, Aiello A (eds) Insects of Panama and Mesoamerica: selected studies. Oxford University Press, Oxford, UK, pp 421–431
Kinzelbach R (1971) Morphologische Befunde an Fächerflüglern und ihre phylogenetische Bedeutung (Insecta: Strepsiptera). In: Schaller F (ed) Zoologica 41 (119). Stuttgart, pp 1–256
Kinzelbach RK (1990) The systematic position of Strepsiptera (Insecta). Am Entomol 35:292–303
Land MF (1981) Optics and vision in invertebrates. In: Autrum H (ed) Handbook of sensory physiology, VII/6B. Springer, Berlin Heidelberg New York, pp 471–592
Laughlin SB, Steveninck RRD van, Anderson JC (1998) The metabolic cost of neural information. Nat Neurosci 1:36–41
Leutscher-Hazelhoff JT, Kuiper J (1964) Response of the blowfly (Calliphora erythrocephala) to light flashes and the sinusoidally modulated light. Doc Ophthalmol 18:275–283
Lin RC, Schneller RH (2000) Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol 16:19–42
Loew ER (1975) CO2-induced changes in the ERG of the fly, Sarcophaga bullata. A component analysis. J Insect Physiol 21:181–197
MacCarthy HR (1991) Compound eye of male Stylops pacifica (Strepsiptera; Stylopidae). J Entomol Soc B C 88:27–31
Matteson N, Terry I, Ascoli-Christensen A, Gilbert C (1992) Spectral efficiency of the western flower thrips, Frankliniella occidentalis. J Insect Physiol 38:453–459
Meinertzhagen IA, Hanson TE (1993) The development of the optic lobe. In: Bate M, Martinez Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, New York, pp 1363–1492
Meinertzhagen IA, Emsley JG, Sun XJ (1998) Developmental anatomy of the Drosophila brain: neuroanatomy is gene expression. J Comp Neurol 402:1–9
Meixner J (1936) 19. Ordnung der Pterygogenea: Strepsiptera Kirby (1813)=Fächerflügler oder Kolbenflügler. In: Krumbach T (ed) Handbuch der Zoologie 4, Band 2. Hälfte, erster Teil, Insecta 2, pp 1349–1382
Mizutani A, Toh Y (1995) Optical and physiological properties of the larval visual system of the tiger beetle Cicindela chinensis. J Comp Physiol A 177:591–599
Nilsson D-E (1989) The evolution of compound eyes. In: Stavenga DG, Hardie RC (eds) Facets of vision. Springer, Berlin Heidelberg New York, pp 30–73
Nilsson and Osorio (1997)
Okamura J-Y, Toh Y (2001) Responses of medulla neurons to illuminations and movement stimuli in the tiger beetle larvae. J Comp Physiol A 187:713–725
Paulus HF (1979) Eye structure and the monophyly of the Arthropoda. In: Gupta AP (ed) Arthropod phylogeny. Van Nostrand Reinhold, New York, pp 299–383
Paulus HF (2000) Phylogeny of the Myriapoda-Crustacea-Insecta: a new attempt using photoreceptor structure. J Zool Syst Evol Res 38:189–208
Perkins RCL (1918) The assembling and pairing of Stylops. Entomol Mon Mag 54:129–131
Pix W, Zanker JM, Zeil J (2000) The optomotor response and spatial resolution of the visual system in male Xenos vesparum (Strepsiptera). J Exp Biol 203:3397–3409
Rokas A, Kathirithamby J, Holland PWH (1999) Intron insertion as a phylogenetic character: the engrailed homeobox of Strepsiptera does not indicate affinity with Diptera. Insect Mol Biol 8:527–530
Rösch P (1913) Beiträge zur Kenntnis der Entwicklungsgeschichte der Strepsipteren. Z Naturwiss 50:97–146
Schuppe H, Hengstenberg R (1993) Optical properties of the ocelli of Calliphora erythrocephala and their role in the dorsal light response. J Comp Physiol A 173:143–149
Stange G, Stowe S, Chahl JS, Massaro A (2002) Anisotropic imaging in the dragonfly median ocellus: a matched filter for horizon detection. J Comp Physiol A 188:455–467
Strausfeld NJ (1970) Golgi studies on insects. II. The optic lobes of Diptera. Philos Trans R Soc Lond B 258:175–223
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York
Strausfeld NJ (1984) Functional neuroanatomy of the blowfly's visual system. In: Ali MA (ed) Photoreception and vision in invertebrates. Plenum Press, New York, pp 483–522
Strausfeld NJ, Lee J-K (1991) Neuronal basis for parallel visual processing in the fly. Vis Neurosci 7:13–33
Strausfeld NJ, Seyan HS (1985) Convergence of visual, haltere, and prosternal inputs at neck motor neurons of Calliphora erythrocephala. Cell Tissue Res 247:5–10
Strohm K (1910) Die zusammengesetzten Augen der Männchen von Xenos rossii. Zool Anz 36:156–159
Toh Y, Mizutani A (1994a) Structure of the visual system of the larva of the tiger beetle (Cicindela chinensis). Cell Tissue Res 278:125–134
Toh Y, Mizutani A (1994b) Neural organization of the lamina neuropil of he larva of the tiger beetle (Cicindela chinensis). Cell Tissue Res 278:134–144
Wachmann E (1971)
Wachmann E (1972) Fine-structure of compound eye of Stylops spec. (Insecta, Strepsiptera). Z Zellforsch Mikroskop Anat 123:411–
Wheeler WC, Whiting M, Wheeler QD, Carpenter JM (2001) The phylogeny of the extant hexapod orders. Cladistics 17:113–169
Whiting MF (1998) Phylogenetic position of the Strepsiptera: review of molecular and morphological evidence. Int J Insect Morphol Embryol 27:53–60
Whiting MF, Carpenter JC, Wheeler QD, Wheeler WC (1997) The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst Biol 46:1–68
Wilson M (1978) Functional organization of locust ocelli. J Comp Physiol 124:297–316
Zeil J (1983) Sexual dimorphism in the visual system of flies: the divided brain of male Bibionidae (Diptera). Cell Tissue Res 229:591–610
Acknowledgements
We thank H. Howland C. Gilbert, and Mike Land for helpful discussions and all members of the Hoy laboratory and Ilya Vilinsky for their comments on the manuscript. The antibody against synaptotagmin was kindly provided by Dr. Hugo Bellen. This project was funded by the NIH(2R01DC00103) and the NSF(IBN-9974512 and IBN-211770).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buschbeck, E.K., Ehmer, B. & Hoy, R.R. The unusual visual system of the Strepsiptera: external eye and neuropils. J Comp Physiol A 189, 617–630 (2003). https://doi.org/10.1007/s00359-003-0443-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-003-0443-x