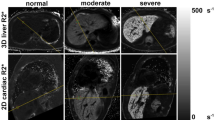
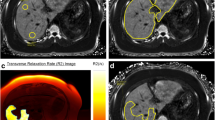
Abstract
Objectives
MRI-based R2* mapping may enable reliable and rapid quantification of liver iron concentration (LIC). However, the performance and reproducibility of R2* across acquisition protocols remain unknown. Therefore, the objective of this work was to evaluate the performance and reproducibility of complex confounder-corrected R2* across acquisition protocols, at both 1.5 T and 3.0 T.
Methods
In this prospective study, 40 patients with suspected iron overload and 10 healthy controls were recruited with IRB approval and informed written consent and imaged at both 1.5 T and 3.0 T. For each subject, acquisitions included four different R2* mapping protocols at each field strength, and an FDA-approved R2-based method performed at 1.5 T as a reference for LIC. R2* maps were reconstructed from the complex data acquisitions including correction for noise effects and fat signal. For each subject, field strength, and R2* acquisition, R2* measurements were performed in each of the nine liver Couinaud segments and the spleen. R2* measurements were compared across protocols and field strength (1.5 T and 3.0 T), and R2* was calibrated to LIC for each acquisition and field strength.
Results
R2* demonstrated high reproducibility across acquisition protocols (p > 0.05 for 96/108 pairwise comparisons across 2 field strengths and 9 liver segments, ICC > 0.91 for each field strength/segment combination) and high predictive ability (AUC > 0.95 for four clinically relevant LIC thresholds). Calibration of R2* to LIC was LIC = − 0.04 + 2.62 × 10−2 R2* at 1.5 T and LIC = 0.00 + 1.41 × 10−2 R2* at 3.0 T.
Conclusions
Complex confounder-corrected R2* mapping enables LIC quantification with high reproducibility across acquisition protocols, at both 1.5 T and 3.0 T.
Key Points
• Confounder-corrected R2* of the liver provides reproducible R2* across acquisition protocols, including different spatial resolutions, echo times, and slice orientations, at both 1.5 T and 3.0 T.
• For all acquisition protocols, high correlation with R2-based liver iron concentration (LIC) quantification was observed.
• The calibration between confounder-corrected R2* and LIC, at both 1.5 T and 3.0 T, is determined in this study.






Similar content being viewed by others
Abbreviations
- AA:
-
Aplastic anemia
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- CSE:
-
Chemical shift-encoded
- GRE:
-
Gradient-recalled echo
- HH:
-
Hereditary hemochromatosis
- IRB:
-
Institutional review board
- LIC:
-
Liver iron concentration
- MDS:
-
Myelodysplastic syndrome
- NOS:
-
Not otherwise specified
- SCD:
-
Sickle cell disease
- TH:
-
Transfusional hemosiderosis
References
Pietrangelo A (2004) Hereditary hemochromatosis--a new look at an old disease. N Engl J Med 350:2383–2397
Edwards CQ, Ajioka RS, Kushner JP (2000) Hemochromatosis: a genetic definition. In: Barton JC, Edwards CQ (eds) Hemochromatosis: genetics, pathophysiology, diagnosis and treatmen. Cambridge University Press, Cambridge, pp 8–11
Angastiniotis M, Modell B (1998) Global epidemiology of hemoglobin disorders. Ann N Y Acad Sci 850:251–269
Hassell KL (2010) Population estimates of sickle cell disease in the U.S. Am J Prev Med 38:S512–S521
Chernoff AI (1959) The distribution of the thalassemia gene: a historical review. Blood 14:899–912
Mahesh S, Ginzburg Y, Verma A (2008) Iron overload in myelodysplastic syndromes. Leuk Lymphoma 49:427–438
Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23:1631–1634
Brittenham GM, Cohen AR, McLaren CE et al (1993) Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. Am J Hematol 42:81–85
Alustiza JM, Castiella A, De Juan MD, Emparanza JI, Artetxe J, Uranga M (2007) Iron overload in the liver diagnostic and quantification. Eur J Radiol 61:499–506
Wood JC (2007) Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol 14:183–190
Sirlin CB, Reeder SB (2010) Magnetic resonance imaging quantification of liver iron. Magn Reson Imaging Clin N Am 18(359–381):ix
Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G (1985) Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med 313:1256–1262
Au WY, Lam WW, Chu W et al (2008) A T2* magnetic resonance imaging study of pancreatic iron overload in thalassemia major. Haematologica 93:116–119
Wood JC, Noetzl L, Hyderi A, Joukar M, Coates T, Mittelman S (2010) Predicting pituitary iron and endocrine dysfunction. Ann N Y Acad Sci 1202:123–128
Anderson LJ, Holden S, Davis B et al (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22:2171–2179
Adams P, Brissot P, Powell LW (2000) EASL international consensus conference on Haemochromatosis. J Hepatol 33:485–504
Porter JB (2001) Practical management of iron overload. Br J Haematol 115:239–252
Vichinsky E (2008) Oral iron chelators and the treatment of iron overload in pediatric patients with chronic anemia. Pediatrics 121:1253–1256
Harmatz P, Butensky E, Quirolo K et al (2000) Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 96:76–79
Brittenham GM, Badman DG, National Institute of D, Digestive, Kidney Diseases W (2003) Noninvasive measurement of iron: report of an NIDDK workshop. Blood 101:15–19
Karam LB, Disco D, Jackson SM et al (2008) Liver biopsy results in patients with sickle cell disease on chronic transfusions: poor correlation with ferritin levels. Pediatr Blood Cancer 50:62–65
Nielsen P, Gunther U, Durken M, Fischer R, Dullmann J (2000) Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. J Lab Clin Med 135:413–418
Angelucci E, Brittenham GM, McLaren CE et al (2000) Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med 343:327–331
Siegelman ES, Mitchell DG, Semelka RC (1996) Abdominal iron deposition: metabolism, MR findings, and clinical importance. Radiology 199:13–22
Emond MJ, Bronner MP, Carlson TH, Lin M, Labbe RF, Kowdley KV (1999) Quantitative study of the variability of hepatic iron concentrations. Clin Chem 45:340–346
Villeneuve JP, Bilodeau M, Lepage R, Cote J, Lefebvre M (1996) Variability in hepatic iron concentration measurement from needle-biopsy specimens. J Hepatol 25:172–177
Guyader D, Gandon Y, Robert JY et al (1992) Magnetic resonance imaging and assessment of liver iron content in genetic hemochromatosis. J Hepatol 15:304–308
Dixon RM, Styles P, Al-Refaie FN et al (1994) Assessment of hepatic iron overload in thalassemic patients by magnetic resonance spectroscopy. Hepatology 19:904–910
Gandon Y, Guyader D, Heautot JF et al (1994) Hemochromatosis: diagnosis and quantification of liver iron with gradient-echo MR imaging. Radiology 193:533–538
Henninger B, Alustiza J, Garbowski M, Gandon Y (2020) Practical guide to quantification of hepatic iron with MRI. Eur Radiol 30:383–393
St Pierre TG, Clark PR, Chua-anusorn W et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105:855–861
Wood JC, Enriquez C, Ghugre N et al (2005) MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106:1460–1465
Vasanawala SS, Yu H, Shimakawa A, Jeng M, Brittain JH (2012) Estimation of liver T*2 in transfusion-related iron overload in patients with weighted least squares T*2 IDEAL. Magn Reson Med 67:183–190
Hernando D, Kramer HJ, Reeder SB (2013) Multipeak fat-corrected complex R2* relaxometry: theory, optimization, and clinical validation. Magn Reson Med 70:1319–1331
Ahmed A, Wong RJ, Harrison SA (2015) Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol 13:2062–2070
Reeder SB, Sirlin CB (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 18(337–357):ix
Fernandez-Seara MA, Wehrli FW (2000) Postprocessing technique to correct for background gradients in image-based R*(2) measurements. Magn Reson Med 44:358–366
Hernando D, Vigen KK, Shimakawa A, Reeder SB (2012) R2* mapping in the presence of macroscopic B(0) field variations. Magn Reson Med 68:830–840
Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB (2008) Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 60:1122–1134
Jhaveri KS, Kannengiesser SAR, Ward R, Kuo K, Sussman MS (2019) Prospective evaluation of an R2* method for assessing liver iron concentration (LIC) against FerriScan: derivation of the calibration curve and characterization of the nature and source of uncertainty in the relationship. J Magn Reson Imaging 49:1467–1474
Hernando D, Cook RJ, Diamond C, Reeder SB (2013) Magnetic susceptibility as a B0 field strength independent MRI biomarker of liver iron overload. Magn Reson Med 70:648–656
Sharma SD, Hernando D, Horng DE, Reeder SB (2015) Quantitative susceptibility mapping in the abdomen as an imaging biomarker of hepatic iron overload. Magn Reson Med 74:673–683
Horng DE, Hernando D, Reeder SB (2017) Quantification of liver fat in the presence of iron overload. J Magn Reson Imaging 45:428–439
Meisamy S, Hines CD, Hamilton G et al (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258:767–775
Campo CA, Hernando D, Schubert T, Bookwalter CA, Pay AJV, Reeder SB (2017) Standardized approach for ROI-based measurements of proton density fat fraction and R2* in the liver. AJR Am J Roentgenol 209:592–603
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bassett ML, Halliday JW, Powell LW (1986) Value of hepatic iron measurements in early hemochromatosis and determination of the critical iron level associated with fibrosis. Hepatology 6:24–29
Olivieri NF, Brittenham GM (1997) Iron-chelating therapy and the treatment of thalassemia. Blood 89:739–761
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK (2007) R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. J Magn Reson Imaging 25:540–547
Meloni A, Positano V, Keilberg P et al (2012) Feasibility, reproducibility, and reliability for the T*2 iron evaluation at 3 T in comparison with 1.5 T. Magn Reson Med 68:543–551
Ghugre NR, Doyle EK, Storey P, Wood JC (2015) Relaxivity-iron calibration in hepatic iron overload: predictions of a Monte Carlo model. Magn Reson Med 74:879–883
Krafft AJ, Loeffler RB, Song R et al (2017) Quantitative ultrashort echo time imaging for assessment of massive iron overload at 1.5 and 3 Tesla. Magn Reson Med 78:1839–1851
Doyle EK, Toy K, Valdez B, Chia JM, Coates T, Wood JC (2018) Ultra-short echo time images quantify high liver iron. Magn Reson Med 79:1579–1585
Wiens C, Zhu A, Johnson K, Reeder S, Hernando D (2017) Accuracy and reproducibility of Iron quantification using ultra-short TE imaging at 1.5T and 3.0T Proceedings of the 25th annual meeting of ISMRM, Honolulu, pp 371
Funding
This study has received funding from the WARF Accelerator Program, from the NIH (R01 DK100651, K24 DK102595, R01 DK083380, R01 DK117354), as well as from GE Healthcare who provides research support to UW-Madison. Furthermore, Dr. Reeder is a Romnes Faculty Fellow and has received an award provided from the University of Wisconsin-Madison Office of the Vice-Chancellor of Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Diego Hernando, PhD.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Dr. Hernando is a cofounder of Calimetrix. Dr. Reeder consults for ArTara Therapeutics and HeartVista. Dr. Reeder is a cofounder of Calimetrix.
Statistics and biometry
One of the coauthors (Colin Longhurst, MS, UW Department of Biostatistics and Medical Informatics) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported. Hernando et al (Magnetic resonance in medicine 70 (3), 648–656) demonstrated the feasibility of quantifying liver iron concentration from measured B0 field maps. Sharma et al (Magnetic resonance in medicine 74 (3), 673–683) validated a method for quantitative susceptibility mapping in the liver. Horng et al (Journal of Magnetic Resonance Imaging 45 (2), 428–439) evaluated the accuracy of a single-R2* signal model for fat quantification in the presence of liver iron overload.
Methodology
• prospective
• cross-sectional
• single-institution study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 72 kb)
Rights and permissions
About this article
Cite this article
Hernando, D., Cook, R.J., Qazi, N. et al. Complex confounder-corrected R2* mapping for liver iron quantification with MRI. Eur Radiol 31, 264–275 (2021). https://doi.org/10.1007/s00330-020-07123-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07123-x