Abstract
Objectives
Lung adenocarcinomas which manifest as ground-glass nodules (GGNs) have different degrees of pathological invasion and differentiating among them is critical for treatment. Our goal was to evaluate the addition of marginal features to a baseline radiomics model on computed tomography (CT) images to predict the degree of pathologic invasiveness.
Methods
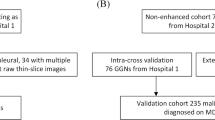
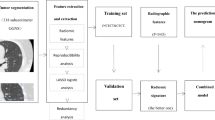
We identified 236 patients from two cohorts (training, n = 189; validation, n = 47) who underwent surgery for GGNs. All GGNs were pathologically confirmed as adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), or invasive adenocarcinoma (IA). The regions of interest were semi-automatically annotated and 40 radiomics features were computed. We selected features using L1-norm regularization to build the baseline radiomics model. Additional marginal features were developed using the cumulative distribution function (CDF) of intratumoral intensities. An improved model was built combining the baseline model with CDF features. Three classifiers were tested for both models.
Results
The baseline radiomics model included five features and resulted in an average area under the curve (AUC) of 0.8419 (training) and 0.9142 (validation) for the three classifiers. The second model, with the additional marginal features, resulted in AUCs of 0.8560 (training) and 0.9581 (validation). All three classifiers performed better with the added features. The support vector machine showed the most performance improvement (AUC improvement = 0.0790) and the best performance was achieved by the logistic classifier (validation AUC = 0.9825).
Conclusion
Our novel marginal features, when combined with a baseline radiomics model, can help differentiate IA from AIS and MIA on preoperative CT scans.
Key Points
• Our novel marginal features could improve the existing radiomics model to predict the degree of pathologic invasiveness in lung adenocarcinoma.



Similar content being viewed by others
Abbreviations
- AIS:
-
Adenocarcinoma in situ
- AUC:
-
Area under the curve
- CDF:
-
Cumulative distribution function
- CT:
-
Computed tomography
- DFS:
-
Disease-free survival
- GGNs:
-
Ground-glass nodules
- GLCM:
-
Gray-level co-occurrence matrix
- HU:
-
Hounsfield unit
- IA:
-
Invasive adenocarcinoma
- ISZM:
-
Intensity size zone matrix
- LASSO:
-
Least absolute shrinkage and selection operator
- MIA:
-
Minimally invasive adenocarcinoma
- MSE:
-
Mean squared error
- RF:
-
Random forest
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
- SVM:
-
Support vector machine
- VNC:
-
Virtual non-contrast-enhanced
References
Austin JHM, Garg K, Aberle D et al (2013) Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology. https://doi.org/10.1148/radiol.12120240
Borczuk AC, Qian F, Kazeros A et al (2009) Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. AmJ Surg Pathol. https://doi.org/10.1097/PAS.0b013e318190157c
Zhang J, Wu J, Tan Q et al (2013) Why do pathological stage IA lung adenocarcinomas vary from prognosis?: a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 8:1196–1202. https://doi.org/10.1097/JTO.0B013E31829F09A7
Kates M, Swanson S, Wisnivesky JP (2011) Survival following lobectomy and limited resection for the treatment of stage I nonsmall cell lung cancer ≤1 cm in size: a review of SEER data. Chest 139:491–496. https://doi.org/10.1378/CHEST.09-2547
Wisnivesky JP, Henschke CI, Swanson S et al (2010) Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. https://doi.org/10.1097/SLA.0b013e3181c0e5f3
MacMahon H, Naidich DP, Goo JM et al (2017) Guidelines for management of incidental pulmonary nodules detected on CT images:from the Fleischner Society 2017. Radiology. https://doi.org/10.1148/radiol.2017161659
Zhang Y, Shen Y, Qiang JW, Ye JD, Zhang J, Zhao RY (2016) HRCT features distinguishing pre-invasive from invasive pulmonary adenocarcinomas appearing as ground-glass nodules. Eur Radiol 26:2921–2928. https://doi.org/10.1007/s00330-015-4131-3
Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK (2007) Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 245:267–275. https://doi.org/10.1148/radiol.2451061682
Yip SS, Aerts HJ (2016) Applications and limitations of radiomics. Phys Med Biol 61:R150–R166. https://doi.org/10.1088/0031-9155/61/13/R150
Prasanna P, Tiwari P, Madabhushi A (2016) Co-occurrence of local anisotropic gradient orientations (CoLlAGe): a new radiomics descriptor. Sci Rep 6:37241. https://doi.org/10.1038/srep37241
Ismail M, Hill V, Statsevych V et al (2018) Shape features of the lesion habitat to differentiate brain tumor progression from pseudoprogression on routine multiparametric MRI: a multisite study. AJNR Am J Neuroradiol 39:2187–2193. https://doi.org/10.3174/ajnr.A5858
Grélard F, Baldacci F, Vialard A, Domenger J-P (2017) New methods for the geometrical analysis of tubular organs. Med Image Anal 42:89–101. https://doi.org/10.1016/J.MEDIA.2017.07.008
Alilou M, Orooji M, Beig N et al (2018) Quantitative vessel tortuosity:a potential CT imaging biomarker for distinguishing lung granulomas from adenocarcinomas. Sci Rep 8:15290. https://doi.org/10.1038/s41598-018-33473-0
Braman NM, Etesami M, Prasanna P et al (2017) Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res 19:57. https://doi.org/10.1186/s13058-017-0846-1
Prasanna P, Patel J, Partovi S et al (2016) Radiomic features from the peritumoral brain parenchyma on treatment-naive multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: preliminary findings. Eur Radiol. https://doi.org/10.1007/s00330-016-4637-3
Naidich DP, Bankier AA, MacMahon H et al (2013) Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 266:304–317. https://doi.org/10.1148/radiol.12120628
Lee SM, Park CM, Song YS et al (2017) CT assessment-based direct surgical resection of part-solid nodules with solid component larger than 5 mm without preoperative biopsy: experience at a single tertiary hospital. Eur Radiol 27:5119–5126. https://doi.org/10.1007/s00330-017-4917-6
Fleiss JL, Levin B, Paik MC (2013) Statistical methods for rates and proportions, 3rd edn. Wiley, Hoboken
UyBico SJ, Wu CC, Suh RD et al (2010) Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 30:1163–1181. https://doi.org/10.1148/rg.305095166
Lee G, Park H, Sohn I et al (2018) Comprehensive computed tomography radiomics analysis of lung adenocarcinoma for prognostication. Oncologist 23:806–813. https://doi.org/10.1634/theoncologist.2017-0538
Van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107. https://doi.org/10.1158/0008-5472.CAN-17-0339
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Syst Man Cybern SMC-3:610–621. https://doi.org/10.1109/TSMC.1973.4309314
Grove O, Berglund AE, Schabath MB et al (2015) Quantitative computed tomographic descriptors associate tumor shape complexity and intratumor heterogeneity with prognosis in lung adenocarcinoma. PLoS One 10:1–14. https://doi.org/10.1371/journal.pone.0118261
Tixier F, Le Rest CC, Hatt M et al (2011) Intratumor heterogeneity characterized by textural features on baseline 18F-FDGPETimages predicts response to concomitant radio chemotherapy in esophageal cancer. J Nucl Med 52:369–378. https://doi.org/10.2967/jnumed.110.082404
Davnall F, Yip CSP, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589. https://doi.org/10.1007/s13244-012-0196-6
de Hoop B, Gietema H, van de Vorst S et al (2010) Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology 255:199–206. https://doi.org/10.1148/radiol.09090571
Lee HY, Jeong JY, Lee KS et al (2012) Solitary pulmonary nodular lung adenocarcinoma: correlation of histopathologic scoring and patient survival with imaging biomarkers. Radiology 264:884–893. https://doi.org/10.1148/radiol.12111793
Tibshirani R (1996) Regression selection and shrinkage via the Lasso. J R Stat Soc B 58:267–288
Son JY, Lee HY, Kim JH et al (2016) Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma:the added value of using iodine mapping. Eur Radiol. https://doi.org/10.1007/s00330-015-3816-y
Nakayama H, Yamada K, Saito H et al (2007) Sublobar resection for patients with peripheral small adenocarcinomas of the lung:surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 84:1675–1679. https://doi.org/10.1016/J.ATHORACSUR.2007.03.015
FangW XY, Zhong C, Chen Q (2014) The IASLC/ATS/ERS classification of lung adenocarcinoma-a surgical point of view. J Thorac Dis 6:S552–S560. https://doi.org/10.3978/j.issn.2072-1439.2014.06.09
Wu T, Dai Y (2017) Tumor microenvironment and therapeutic response. Cancer Lett 387:61–68. https://doi.org/10.1016/J.CANLET.2016.01.043
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322. https://doi.org/10.1016/J.CCR.2012.02.022
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437. https://doi.org/10.1038/nm.3394
Aerts HJWL, Velazquez ER, Leijenaar RTH et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5. https://doi.org/10.1038/ncomms5006
Alcaide-Leon P, Dufort P, Geraldo AF et al (2017) Differentiation of enhancing glioma and primary central nervous system lymphoma by texture-based machine learning. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5173
Kunimatsu A, Kunimatsu N, Kamiya K et al (2018) Comparison between glioblastoma and primary central nervous system lymphoma using MR image-based texture analysis. Magn Reson Med Sci. https://doi.org/10.2463/mrms.mp.2017-0044
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 6:244–285. https://doi.org/10.1097/JTO.0B013E318206A221
Gupta R, Phan CM, Leidecker C et al (2010) Evaluation of dual energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. https://doi.org/10.1148/radiol.10091806
Chae EJ, Song J-W, Seo JB et al (2008) Clinical utility of dualenergy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology. https://doi.org/10.1148/radiol.2492071956
Nomori H, Ohtsuka T, Naruke T, Suemasu K (2003) Histogram analysis of computed tomography numbers of clinical T1 N0 M0 lung adenocarcinoma, with special reference to lymph node metastasis and tumor invasiveness. J Thorac Cardiovasc Surg 126:1584–1589. https://doi.org/10.1016/S0022-5223(03)00885-7
Lee G, Lee HY, Park H et al (2017) Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management:state of the art. Eur J Radiol 86:297–307. https://doi.org/10.1016/J.EJRAD.2016.09.005
Lee AK, DeLellis RA, Silverman ML et al (1990) Prognostic significance of peritumoral lymphatic and blood vessel invasion in node-negative carcinoma of the breast. J Clin Oncol 8:1457–1465. https://doi.org/10.1200/JCO.1990.8.9.1457
Uematsu T (2015) Focal breast edema associated with malignancy on T2-weighted images of breast MRI: peritumoral edema, prepectoral edema, and subcutaneous edema. Breast Cancer 22:66–70. https://doi.org/10.1007/s12282-014-0572-9
Beig N, Khorrami M, Alilou M et al (2019) Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology 290:783–792. https://doi.org/10.1148/radiol.2018180910
Beig N, Patel J, Prasanna P et al (2018) Radiogenomic analysis of hypoxia pathway is predictive of overall survival in Glioblastoma. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-017-18310-0
Fan L, Fang M, Li Z et al (2019) Radiomics signature: a biomarker for the preoperative discrimination of lung invasive adenocarcinoma manifesting as a ground-glass nodule. Eur Radiol 29:889–897. https://doi.org/10.1007/s00330-018-5530-z
Funding
This research was supported by Korea Health Industry Development Institute (HI17C0086), National Research Foundation (NRF-2019R1H1A2079721 and NRF-2017M2A2A7A02018568), Ministry of Science and ICT (IITP-2019-2018-0-01798), IITP grant funded by the AI Graduate School Support Program (No. 2019-0-00421), and Institute for Basic Science (IBS-R015-D1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ho Yun Lee
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohort overlap
Some study subjects have been previously reported in (Son JY, Lee HY, Kim JH, et al (2016) Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol. https://doi.org/10.1007/s00330-015-3816-y.)
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Cho, Hh., Lee, G., Lee, H.Y. et al. Marginal radiomics features as imaging biomarkers for pathological invasion in lung adenocarcinoma. Eur Radiol 30, 2984–2994 (2020). https://doi.org/10.1007/s00330-019-06581-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06581-2