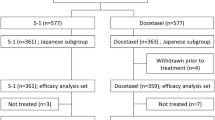
Abstract
Purpose
To gain a better understanding of the impact of dose and other prognostic factors on safety and efficacy of docetaxel in second-line non-small-cell lung cancer patients.
Methods
A model-based meta‐analysis (MBMA) of a published docetaxel monotherapy data in 6085 second‐line non-small-cell lung cancer patients from 46 trials was conducted.
Results
The logit of grade 3/4 neutropenia incidence was a linear function of dose, with a 5 % increase in the odds of neutropenia per mg/m2 increase in dose [odds ratio (OR) 1.05, 95 % confidence interval (CI) 1.04–1.06], and a Japanese study effect (OR 17.1, 95 % CI 6.05–48.4). The logit of overall response rate (ORR) was a linear function of cumulative dose (0.4 % increase in the odds of response per mg/m2 increase; OR 1.004, 95 % CI 1.001–1.008) and median population age (OR 1.08 per year, 95 % CI 1.02–1.15). A Japanese study effect was identified for overall survival (OS) in addition to prognostic factors identified by a previous meta-analysis.
Conclusions
This current MBMA identified docetaxel dose–response relationships for both neutropenia and ORR, an effect of age on ORR, and Japanese study effects on both neutropenia and OS.




Similar content being viewed by others
References
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594. doi:10.4065/83.5.584
Stinchcombe TE, Socinski MA (2008) Considerations for second-line therapy of non-small cell lung cancer. Oncologist 13(Suppl 1):28–36. doi:10.1634/theoncologist.13-S1-28
Mandema JW, Gibbs M, Boyd RA, Wada DR, Pfister M (2011) Model-based meta-analysis for comparative efficacy and safety: application in drug development and beyond. Clin Pharmacol Ther 90(6):766–769. doi:10.1038/clpt.2011.242
Engels FK, Verweij J (2005) Docetaxel administration schedule: from fever to tears? A review of randomised studies. Eur J Cancer (Oxford, England: 1990) 41(8):1117–1126. doi:10.1016/j.ejca.2005.02.016
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16(1):187–196
Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA, Groult V, Murawsky M, Cold S (2006) Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol 24(31):4963–4970. doi:10.1200/jco.2005.05.0294
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4(6):423–436. doi:10.1038/nrc1369
Clarke SJ, Rivory LP (1999) Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 36(2):99–114. doi:10.2165/00003088-199936020-00002
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20(24):4713–4721
Haidich AB (2010) Meta-analysis in medical research. Hippokratia 14(Suppl 1):29–37
Shen G, Bian G, Yu H, Gao M, Kang D, Shen G, Hu S (2014) Comparison between cisplatin plus vinorelbine and cisplatin plus docetaxel in the treatment of advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Mol Clin Oncol 2(1):146–150. doi:10.3892/mco.2013.210
Di BS, Wei KP, Tian JH, Xiao XJ, Li Y, Zhang XH, Yu Q, Yang KH, Ge L, Huang WH, Zhang FW (2014) Effectiveness and safety of pemetrexed versus docetaxel as a treatment for advanced non-small cell lung cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev APJCP 15(8):3419–3424
Li X, Wang H, Lin W, Xu Q (2014) Efficacy of combining targeted therapy with pemetrexed or docetaxel as second-line treatment in patients with advanced non-small-cell lung cancer: a meta-analysis of 14 randomized controlled trials. Curr Med Res Opin. doi:10.1185/03007995.2014.909392
Des Guetz G, Uzzan B, Nicolas P, Valeyre D, Sebbane G, Morere JF (2012) Comparison of the efficacy and safety of single-agent and doublet chemotherapy in advanced non-small cell lung cancer in the elderly: a meta-analysis. Crit Rev Oncol/Hematol 84(3):340–349. doi:10.1016/j.critrevonc.2012.03.007
Qi WX, Shen Z, Yao Y (2012) Meta-analysis of docetaxel-based doublet versus docetaxel alone as second-line treatment for advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 69(1):99–106. doi:10.1007/s00280-011-1678-9
Di Maio M, Chiodini P, Georgoulias V, Hatzidaki D, Takeda K, Wachters FM, Gebbia V, Smit EF, Morabito A, Gallo C, Perrone F, Gridelli C (2009) Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 27(11):1836–1843. doi:10.1200/JCO.2008.17.5844
Di Maio M, Perrone F, Chiodini P, Gallo C, Camps C, Schuette W, Quoix E, Tsai CM, Gridelli C (2007) Individual patient data meta-analysis of docetaxel administered once every 3 weeks compared with once every week second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 25(11):1377–1382. doi:10.1200/JCO.2006.09.8251
Maio M, Lama N, Morabito A, Smit EF, Georgoulias V, Takeda K, Quoix E, Hatzidaki D, Wachters FM, Gebbia V, Tsai CM, Camps C, Schuette W, Chiodini P, Piccirillo MC, Perrone F, Gallo C, Gridelli C (2010) Clinical assessment of patients with advanced non-small-cell lung cancer eligible for second-line chemotherapy a prognostic score from individual data of nine randomised trials. Eur J Cancer (Oxford, England: 1990) 46(4):735–743. doi:10.1016/j.ejca.2009.12.013
Di Maio M, Krzakowski M, Fougeray R, Kowalski DM, Gridelli C (2012) Prognostic score for second-line chemotherapy of advanced non-small-cell lung cancer: external validation in a phase III trial comparing vinflunine with docetaxel. Lung Cancer 77(1):116–120. doi:10.1016/j.lungcan.2012.01.013
Lu D, Joshi A, Li H, Zhang N, Ren MM, Gao Y, Wada R, Jin JY (2014) Model-based meta-analysis for quantifying Paclitaxel dose response in cancer patients. CPT Pharmacomet Syst Pharmacol. doi:10.1038/psp.2014.14
Sally Green JPH, Alderson P, Clarke M, Mulrow CD, Oxman AD (2009) Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JPT, Green s (eds) The cochrane collaborations. Wiley, New York
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed.1000097
Takeda K, Negoro S, Tamura T, Nishiwaki Y, Kudoh S, Yokota S, Matsui K, Semba H, Nakagawa K, Takada Y, Ando M, Shibata T, Saijo N (2009) Phase III trial of docetaxel plus gemcitabine versus docetaxel in second-line treatment for non-small-cell lung cancer: results of a Japan Clinical Oncology Group trial (JCOG0104). Ann Oncol 20(5):835–841. doi:10.1093/annonc/mdn705
Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, Takeda K, Inoue A, Tomii K, Harada M, Masuda N, Jiang H, Itoh Y, Ichinose Y, Saijo N, Fukuoka M (2008) Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26(26):4244–4252. doi:10.1200/JCO.2007.15.0185
Wada R (2013) Technical challenges in modeling Kaplan‐Meier survival curves. In: Paper presented at the American Conference on Pharmacometrics, Ft. Lauderdale, FL, May 2013
Quartino AL, Friberg LE, Karlsson MO (2012) A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Invest New Drugs 30(2):833–845. doi:10.1007/s10637-010-9603-3
Perng RP, Shih JF, Chen YM, Chou KC, Lee YC, Tsai CM (2000) A phase II study of single-agent docetaxel chemotherapy for non-small cell lung cancer. Jpn J Clin Oncol 30(10):429–434
Chen JP, Lo Y, Yu CJ, Hsu C, Shih JY, Yang CH (2008) Predictors of toxicity of weekly docetaxel in chemotherapy-treated non-small cell lung cancers. Lung Cancer 60(1):92–97. doi:10.1016/j.lungcan.2007.09.004
Tsao TC, Chen CH, Chang JW, Lee CH (2006) Weekly short infusion of taxotere at a 4 week cycle in Chinese patients with advanced NSCLC who have failed or relapsed after the frontline platinum-based non-taxane chemotherapy–a Phase II trial. Jpn J Clin Oncol 36(2):80–84. doi:10.1093/jjco/hyi230
Li R, Sun L, Wang J, Qian J, Wang Z, Jiao X (2012) Pemetrexed versus docetaxel in second line non-small-cell lung cancer: results and subsets analyses of a multi-center, randomized, exploratory trial in Chinese patients. Pulm Pharmacol Ther 25(5):364–370. doi:10.1016/j.pupt.2012.06.008
Sun Y, Wu YL, Zhou CC, Zhang L, Zhang L, Liu XY, Yu SY, Jiang GL, Li K, Qin SK, Ma SL, Han L, Quinlivan M, Orlando M, Zhang XQ (2013) Second-line pemetrexed versus docetaxel in Chinese patients with locally advanced or metastatic non-small cell lung cancer: a randomized, open-label study. Lung Cancer 79(2):143–150. doi:10.1016/j.lungcan.2012.10.015
Sirisinha T, Sirilertrakul S, Jirajarus M, Ratanatharathorn V (2005) Doxetaxel in previously treated non-small cell lung cancer patients: clinical efficacy and quality of life. Southeast Asian J Trop Med Public Health 36(1):246–253
Segawa Y, Kiura K, Hotta K, Takigawa N, Tabata M, Matsuo K, Yoshioka H, Hayashi H, Kawai H, Aoe K, Maeda T, Ueoka H, Tanimoto M (2010) A randomized phase II study of a combination of docetaxel and S-1 versus docetaxel monotherapy in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy: results of Okayama Lung Cancer Study Group (OLCSG) Trial 0503. J Thorac Oncol 5(9):1430–1434. doi:10.1097/JTO.0b013e3181e3248e
Kenmotsu H, Tanigawara Y (2015) Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the Western dose. Cancer Sci 106(5):497–504. doi:10.1111/cas.12647
Pallis AG, Karampeazis A, Vamvakas L, Vardakis N, Kotsakis A, Bozionelou V, Kalykaki A, Hatzidaki D, Mavroudis D, Georgoulias V (2011) Efficacy and treatment tolerance in older patients with NSCLC: a meta-analysis of five phase III randomized trials conducted by the Hellenic Oncology Research Group. Ann Oncol 22(11):2448–2455. doi:10.1093/annonc/mdq772
Claret L, Bruno R, Lu JF, Sun YN, Hsu CP (2014) Exploratory modeling and simulation to support development of motesanib in Asian patients with non-small cell lung cancer based on MONET1 study results. Clin Pharmacol Ther 95(4):446–451. doi:10.1038/clpt.2014.11
Ardizzoia A, Acquati M, Fagnani D, Giordano M, Visini M, Scanni A, Quattrone A, Fusco O, Vergani C, Casartelli C, Tagliabue P, Malugani F, Group P (2004) Second line therapy with weekly low-dose docetaxel for pretreated non-small-cell lung carcinoma patients: a multicenter Italian phase II study. Lung 182(1):1–8. doi:10.1007/s00408-003-1039-5
Acknowledgments
We wish to acknowledge Angelica Quartino, Ph.D., for helpful discussion regarding analysis of longitudinal neutrophil count data following docetaxel administration.
Author contributions
MS, MG, EC, and JJ wrote the manuscript. MS, MG, NZ, and RW analyzed the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The study was funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland, and Genentech, Inc., a member of the Roche Group, South San Francisco, CA, USA. MS, EC, and JJ are Genentech employees and own Roche stock. MG, NZ, and RW are Quantitative Solutions employees.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2015_2957_MOESM3_ESM.pdf
Survival curves observed (from Di Maio [18]) and predicted by the Di Maio reference model. The black solid lines connect the observed points. The colored (dashed or solid) lines are predictions from the reference model (PDF 103 kb)
Rights and permissions
About this article
Cite this article
Stroh, M., Green, M., Cha, E. et al. Meta-analysis of published efficacy and safety data for docetaxel in second-line treatment of patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 77, 485–494 (2016). https://doi.org/10.1007/s00280-015-2957-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2957-7