Abstract
Purpose
We designed this study in locally advanced rectal cancer to determine the pathological response, toxicity, and disease-free survival (DFS) with induction capecitabine plus irinotecan followed by capecitabine-based chemoradiotherapy (CRT) and analyze the gene expression of enzymes involved in the metabolism of capecitabine and irinotecan for associations with response and toxicity.
Methods
Patients with T3/T4 or node positive rectal cancer were treated with capecitabine 1,000 mg/m2 twice daily (BID) days 1–14, and irinotecan 200 mg/m2 on day 1 every 21 days for 2 cycles, followed by capecitabine 825 mg/m2 BID days 1–5 per week with concurrent radiotherapy 50.4 Gy in 28 fractions. Surgical resection occured a median of 7.4 weeks after CRT. Gene expression levels or sequencing were used to analyze carboxylesterase-converting enzymes (CES1, CES2), thymidylate synthase (TS), thymidine phosphorylase (TP), dehydropyrimidine dehydrogenase (DPD), topoisomerase I (TOPO I), and uridine-diphosphate (UDP) glucuronosyl transferase 1A1 in pre- and post-treatment tumor and normal tissue samples.
Results
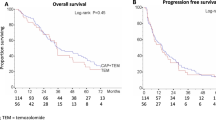
Twenty-two patients were enrolled, and 18 completed neoadjuvant therapy and underwent R0 resection. Two patients with UGT1A1 7/7 had grade 3 and 4 neutropenic fever and sepsis. Pathological complete response (pCR) occurred in 6 of 18 patients (33 %) and 10 (56 %) had tumor and/or nodal downstaging. The 3-year DFS was 75.5 % (95 % CI, 39.7–91.8 %). Locoregional control rate was 100 %. We observed higher TP gene expression in pCR patients, but no correlations with toxicity.
Conclusions
This neoadjuvant regimen was safe and demonstrated significant antitumor activity. High TP tumor gene expression was associated with obtaining pCR.




Similar content being viewed by others
References
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Gerard JP, Conroy T, Bonnetain F et al (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24:4620–4625
Roh MS, Colangelo LH, O’Connell MJ et al (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27:5124–5130
Fernandez-Martos C, Pericay C, Aparicio J et al (2010) Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 28:859–865
Meropol NJ, Gold PJ, Diasio RB et al (2006) Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 24:4069–4077
Schiel MA, Green SL, Davis WI et al (2007) Expression and characterization of a human carboxylesterase 2 splice variant. J Pharmacol Exp Ther 323:94–101
Monaghan G, Ryan M, Seddon R et al (1996) Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 347:578–581
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151
Crane CH, Eng C, Feig BW et al (2010) Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 76:824–830
Horisberger K, Treschl A, Mai S et al (2009) Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a Phase II MARGIT trial. Int J Radiat Oncol Biol Phys 74:1487–1493
Gerard JP, Azria D, Gourgou-Bourgade S et al (2010) Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 28:1638–1644
Meropol NJ (1998) Oral fluoropyrimidines in the treatment of colorectal cancer. Eur J Cancer 34:1509–1513
Salonga D, Danenberg KD, Johnson M et al (2000) Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res 6:1322–1327
Koopman M, Venderbosch S, Van Tinteren H et al (2009) Predictive and prognostic markers for the outcome of chemotherapy in advanced colorectal cancer, a retrospective analysis of the phase III randomized CAIRO study. Eur J Cancer 45:1999–2006
Toi M, Atiqur Rahman M, Bando H et al (2005) Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol 6:158–166
Kim TD, Li G, Song KS et al (2009) Radiation-induced thymidine phosphorylase upregulation in rectal cancer is mediated by tumor-associated macrophages by monocyte chemoattractant protein-1 from cancer cells. Int J Radiat Oncol Biol Phys 73:853–860
Braun MS, Richman SD, Quirke P et al (2008) Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol 26:2690–2698
Horisberger K, Erben P, Muessle B et al (2009) Topoisomerase I expression correlates to response to neoadjuvant irinotecan-based chemoradiation in rectal cancer. Anticancer Drugs 20:519–524
Johnston SJ, Ridge SA, Cassidy J et al (1999) Regulation of dihydropyrimidine dehydrogenase in colorectal cancer. Clin Cancer Res 5:2566–2570
Ulukan H, Muller MT, Swaan PW (2001) Downregulation of topoisomerase I in differentiating human intestinal epithelial cells. Int J Cancer 94:200–207
Sanghani SP, Sanghani PC, Schiel MA et al (2009) Human carboxylesterases: an update on CES1, CES2 and CES3. Protein Pept Lett 16:1207–1214
Quinney SK, Sanghani SP, Davis WI et al (2005) Hydrolysis of capecitabine to 5′-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide. J Pharmacol Exp Ther 313:1011–1016
Sanghani SP, Quinney SK, Fredenburg TB et al (2004) Hydrolysis of irinotecan and its oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin and 7-ethyl-10-[4-(1-piperidino)-1-amino]-carbonyloxycamptothecin, by human carboxylesterases CES1A1, CES2, and a newly expressed carboxylesterase isoenzyme, CES3. Drug Metab Dispos 32:505–511
Tanimoto K, Kaneyasu M, Shimokuni T et al (2007) Human carboxylesterase 1A2 expressed from carboxylesterase 1A1 and 1A2 genes is a potent predictor of CPT-11 cytotoxicity in vitro. Pharmacogenet Genom 17:1–10
Javle MM, Yang G, Nwogu CE et al (2009) Capecitabine, oxaliplatin and radiotherapy: a phase IB neoadjuvant study for esophageal cancer with gene expression analysis. Cancer Invest 27:193–200
Acknowledgments
Funding for this study was provided by Roche Pharmaceuticals and Pfizer Pharmaceuticals.
Conflict of interest
No conflict of interest exists for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chiorean, E.G., Sanghani, S., Schiel, M.A. et al. Phase II and gene expression analysis trial of neoadjuvant capecitabine plus irinotecan followed by capecitabine-based chemoradiotherapy for locally advanced rectal cancer: Hoosier Oncology Group GI03-53. Cancer Chemother Pharmacol 70, 25–32 (2012). https://doi.org/10.1007/s00280-012-1883-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1883-1