Abstract
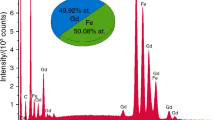
High-temperature X-ray powder-diffraction study of astrophyllite, K2NaFe7 2+Ti2(Si4O12)2O2(OH)4F, and investigation of the samples annealed at 600 and 700 °C, reveal the occurrence of a phase transformation due to the thermal iron oxidation coupled with (1) deprotonation according to the scheme Fe2+ + OH− → Fe3+ + O2− + ½H2 ↑, and (2) defluorination according to the scheme Fe2+ + F− → Fe3+ + O2−. The phase transformation occurs at 500 °C, it is irreversible and without symmetry changes. The mineral decomposes at 775 °C. Both astrophyllite and its high-temperature dehydroxylated (HT) modification are triclinic, P-1. The unit-cell parameters are a = 5.3752(1), b = 11.8956(3), c = 11.6554(3) Å, α = 113.157(3), β = 94.531(2), γ = 103.112(2)º, V = 655.47(3) Å3 for unheated astrophyllite, and a = 5.3287(4), b = 11.790(1), c = 11.4332(9) Å, α = 112.530(8), β = 94.539(6), γ = 103.683(7)º, V = 633.01(9) Å3 for the HT (annealed) modification of astrophyllite. The oxidation of iron is confirmed: (1) by the presence of an exothermic effect at 584 °C in the DTA/TG curves in an Ar–O atmosphere and its absence in an Ar–Ar atmosphere and (2) by ex situ Mössbauer spectroscopy that showed the oxidation of Fe2+ to Fe3+ in the samples heated to 700 °C. Deprotonation was detected by the evolution of IR spectra in the region 3600–3000 cm−1 for astrophyllite and its HT modification. Defluorination was detected by the presence of F in the electron microprobe analysis of unheated astrophyllite and the absence of F in the analysis of unpolished heated astrophyllite. The significant difference between astrophyllite and its HT modification is in the reduction of the M–O interatomic distances after heating to 500 °C and the distortion indices of the MO6 and Dφ6 octahedra. Thermal behaviour of astrophyllite in the 25–475 °C temperature range can be described as a volume thermal expansion with maximal coefficient of thermal expansion in the direction perpendicular to the plane of the HOH layers. In contrast, the HT phase experiences a strong contraction in the 600–775 °C temperature range, again in the direction perpendicular to the plane of the HOH layers.








Similar content being viewed by others
References
Agakhanov AA, Pautov LA, Uvarova YuA, Sokolova E, Hawthorne FC, Karpenko VY (2008) Nalivkinite, Li2NaFe2+ 7Ti2 (Si8O24)O2(OH)4F, a new mineral of the astrophyllite group from the Darai-Pioz Massif, Tadjikistan. New Data Miner 43:5–12
Agakhanov AA, Pautov LA, Sokolova E, Abdu YA, Hawthorne FC, Karpenko VY (2016) Two astrophyllite-supergroup minerals, bulgakite and nalivkinite: bulgakite, a new mineral from the Darai-Pioz alkaline massif, Tajikistan and revision of the crystal structure and chemical formula of nalivkinite. Can Miner 54:3–48
Bačik P, Ozdin D, Miglierini M, Kardošová P, Pentrák M, Haloda J (2011) Crystallochemical effects of heat treatment on Fe-dominant tourmalines from Dolni Bory (Czech Republic) and Vlachovo(Slovakia). Phys Chem Miner 38:599–611
Baur WH (1974) The geometry of polyhedral distortions. Predictive relationships for the phosphate group. Acta Cryst B 30:1195–1215
Bayliss P (2007) Cesium kupletskite renamed kupletskite-(Cs). Mineral Mag 71:365–367
Belousov R, Filatov S (2007) Algorithm for calculating the thermal expansion tensor and constructing the thermal expansion diagram for crystals. Glass Phys Chem 33(3):271–275
Brindley GW, Lemaitre J (1987) Thermal, oxidation and reduction reactions of clay minerals. In Newman ACD (ed) Chemistry of clay and clay minerals. Monograph, Mineralogical Society, pp 319–370
Brown ID (2009) Recent developments in the methods and applications of the bond valence model. Chem Rev 109:6858–6919
Bruker AXS (2009) Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data. Karlsruhe, Germany
Bruker-AXS (2014) APEX2. Version 2014.11–0. Madison, Wisconsin, USA
Bubnova RS, Firsova VA, Filatov SK (2013) Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (theta to tensor-TTT). Glass Phys Chem 39(3):347–350
Cámara F, Sokolova E, Abdu Y, Hawthorne FC (2010) The crystal structures of niobophyllite, kupletskite-(Cs) and Sn-rich astrophyllite: revisions to the crystal chemistry of the astrophyllite-group minerals. Can Miner 48:1–16
Cámara F, Sokolova E, Abdu Y, Hawthorne FC (2014) Nafertisite, Na3Fe2+ 10Ti2(Si6O17)2O2(OH)6F(H2O)2, from Mt. Kukisvumchorr, Khibiny alkaline massif, Kola peninsula, Russia: Refinement of the crystal structure and revision of the chemical formula. Eur J Miner 26:689–700
Caucia F, Callegari A, Oberti R, Lingaretti O, Hawthorne FC (1994) Structural aspects of oxidation-dehydrogenation in staurolite. Can Mineral 32:477–489
Chon C-M, Lee C-K, Song Y, Kim SA (2006) Structural changes and oxidation of ferroan phlogopite with increasing temperature: in situ neutron powder diffraction and Fourier transform infrared spectroscopy. Phys Chem Miner 33:289–299
Della Ventura G (2015) FTIR spectroscopy at HT: applications and problems. Period Miner ECMS 2015:7–8
Donnay G, Morimoto N, Takeda H (1964) Trioctahedral one-layer micas. II. Prediction of the structure from composition and cell dimensions. Acta Cryst 17:1374–1381
Ferrow EA, Annersten H, Gunawardane RP (1988) Mössbauer effect study on the mixed valence state of iron in tourmaline. Miner Mag 52:221–228
Filip J, Bosi F, Novák M, Skogby H, Tuček J, Čuda J, Wildner M (2012) Iron redox reactions in the tourmaline structure: High-temperature treatment of Fe3+-rich schorl. Geochim Cosmochim Acta 86:239–256
Génin JMR, Guérin O, Herbillon AJ, Kuzmann E, Mills SJ, Morin G, Ona-Nguema G, Ruby C, Upadhyay C (2013) Redox topotactic reactions in FeII–III(oxy)hydroxycarbonate new minerals related to fougèrite in gleysols: «trébeurdenite and mössbauerite». Hyperfine Interact 204:71–81
Génin JMR, Mills SJ, Christy AG, Guérin O, Herbillon AJ, Kuzmann E, Ona-Nguema G, Ruby C, Upadhyay C (2014a) Mössbauerite, Fe3+ 6O4(OH)8[CO3]·3H2O, the fully oxidized ‘green rust’ mineral from Mont Saint-Michel Bay, France. Miner Mag 78:447–465
Génin JMR, Christy A, Kuzmann E, Mills S, Ruby C (2014b) Structure and occurrences of <green rust> related new minerals of the <fougérite> group, trébeurdenite and mössbauerite, belonging to the <hydrotalcite> supergroup; how Mössbauer spectroscopy helps XRD. Hyperfine Interact 226:459–482
Güttler B, Niemann W, Redfern (1989) S.A.T. EXAFS and XANES spectroscopy study of the oxidation and deprotonation of biotite. Miner Mag 53:591–602
Hazen RM, Downs RT, Prewitt CT (2000) Principles of comparative crystal chemistry. In: Hazen RM, Downs RT (eds) Reviews in mineralogy and geochemistry, high-temperature and high-pressure crystal chemistry, vol 14. Mineralogical Society of America, Washington, pp 1–33
Kampf AR, Rossman GR, Steele IM, Pluth JJ, Dunning GE, Walstrom RE (2010) Devitoite, a new heterophyllosilicate mineral with astrophyllite-like layers from Eastern Fresno county, California. Can Miner 48:29–40
Kapustin YL (1972) Zircophyllite—the zirconium analogue of astrophyllite. Zap Vses Miner Obshchest 101(4):459–463 (in Russian)
Kapustin YL (1973) Zircophyllite, the zirconium analog of astrophyllite. Int Geol Rev 15:621–625
Khomyakov AP, Cámara F, Sokolova E, Abdu Y, Hawthorne FC (2011) Sveinbergeite, Ca(Fe2+ 6Fe3+)Ti2(Si4O12)2O2(OH)5(H2O)4, a new astrophyllite-group mineral from the Larvik Plutonic Complex, Oslo Region, Norway: description and crystal structure. Miner Mag 75:2687–2702
Korovushkin VV, Kuzmin V, Belov VF (1979) Mossbauer studies of structural features in tourmaline of various genesis. Phys Chem Miner 4:209–220
Kunz M, Brown ID (1994) Out-of-center distortions around octahedrally coordinated d0-transition metals. J Solid State Chem 115:395–406
Langreiter T, Kahlenberg V (2014) TEV—a program for the determination and visualization of the thermal expansion tensor from diffraction data. Institute of Mineralogy and Petrography, University of Innsbruck, Austria
Lepp H (1957) Stages in the oxidation of magnetite. Am Miner 42:679–681
Liebau F (1985) Structural chemistry of silicates: structure, bonding and classification. Springer-Verlag, Berlin
Mills SJ, Christy AG, Génin JMR, Kameda T, Colombo F (2012) Nomenclature of the hydrotalcite supergroup: natural layered double hydroxides. Miner Mag 76:1289–1336
Momma K, Izumi F (2011) Vesta 3 for free-dimensional visualization of crystals, volumetric and morphology data. J Appl Crystallogr 44:1272–1276
Murad E, Wagner U (1996) The thermal behaviuor of an Fe-rich illite. Clay Miner 31:45–52
Nickel EH, Rowland JF, Charette DJ (1964) Niobophyllite—the niobium analogue of astrophyllite; a new mineral from Seal Lake, Labrador. Can Miner 8:40–52
Oberti R, Della Ventura G, Dyar MD (2015) Combining structure refinement and spectroscopies: hints and warnings for more efficient tolls to decipher the mechanism of deprotonation in amphiboles. Period Miner ECMS 2015:131–132
Piilonen PC, Lalonde AE, Mcdonald AM, Gault RA (2000) Niobokupletskite, a new astrophyllite-group mineral from Mont Saint-Hilaire, Quebec, Canada: description and crystal structure. Can Miner 38:627–639
Piilonen PC, McDonald AM, LaLonde AE (2001) Kupletskite polytypes from the Lovozero massif, Kola Peninsula, Russia: Kupletskite-1A and kupletskite-Ma2b2c. Eur J of Miner 13:973–984
Piilonen PC, LaLonde AE, McDonald AM, Gault RA, Larsen AO (2003a) Insights into astrophyllite–group minerals. I. Nomenclature, composition and development of a standardized general formula. Can Miner 41:1–26
Piilonen PC, McDonald AM, LaLonde AE (2003b) Insights into astrophyllite–group minerals. II. Crystal chemistry. Can Miner 41:27–54
Pillonen PC, Pekov IV, Back M, Steede T, Gault RA (2006) Crystal-structure refinement of a Zn-rich kupletskite from Mont Saint-Hilaire, Quebec, with contributions to the geochemistry of zinc in peralkaline environments. Miner Mag 70:565–578
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172(3983):567–570
Russell RL, Guggenheim S (1999) Crystal structures of near-end-member phlogopite at high temperatures and heat-treated Fe-rich phlogopite: the influence of the O, OH, F site. Can Miner 37:711–729
Semenov EI (1956) Kupletskite—a new mineral of the astrophyllite group. Doklady Akademii Nauk SSSR 108:933–936
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Shi N, Ma Z, Li G, Yamnova NA, Pushcharovsky DY (1998) Structure refinement of monoclinic astrophyllite. Acta Crystallogr B 54:109–114
Sokolova E (2012) Further developments in the structure topology of the astrophyllite-group minerals. Miner Mag 76:863–882
Sokolova E, Cámara F (2008) Re-investigation of the crystal structure of magnesium astrophyllite. Eur J Miner 20:253–260
Sokolova E, Hawthorne FC (2016) The crystal structure of zircophyllite, K2NaFe2+ 7Zr2(Si4O12)2O2(OH)4F, an astrophyllite-supergroup mineral from Mont Saint-Hilaire, Québec, Canada. Can Miner (in press)
Sokolova E, Cámara F, Hawthorne FC, Cirotti M (2017a) The astrophyllite supergroup: nomenclature and classification. Miner Mag 81:143–150
Sokolova E, Cámara F, Hawthorne FC, Semenov EI, Cirotti M (2017b) Lobanovite K2Na(Fe2+ 4Mg2Na)Ti2(Si4O12)2O2(OH)4, a new mineral of astrophyllite supergroup and its relation to magnesioastrophyllite. Miner Mag 81:175–181
Stepanov AV, Bekenova GK, Levin VL, Sokolova E, Hawthorne FC, Dobrovol’skaya EA (2012) Tarbagataite, (K,□)2(Ca,Na)(Fe2+ Mn)7Ti2(Si4O12)2O2(OH)4(OH, F), a new astrophyllite-group mineral species from the Verkhnee Espe Deposit, Akjailyautas Mountains, Kazakhstan: description and crystal structure. Can Miner 50:159–168
Susta U, Della Ventura G, Bellatreccia F, Hawthorne FC, Oberti R (2015) HT-FTIR spectroscopy of riebeckite. Period Miner ECMS 2015:167–168
Tutti F, Dubrovinsky LS, Nygren M (2000) High-temperature study and thermal expansion of phlogopite. Phys Chem Miner 27:599–603
Uvarova YA, Sokolova E, Hawthorne FC, Agakhanov AA, Pautov LA (2008) The crystal structure of nalivkinite, a new lithium member of the astrophyllite group. Can Miner 46:651–659
Veith JA, Jackson ML (1974) Iron oxidation and reduction effects on structural hydroxyl and layer charge in aqueous suspensions of micaceous vermiculites. Clays Clay Miner 22:345–353
Ventruti G, Zema M, Scordari F, Pedrazzi G (2008) Thermal behavior of a Ti-rich phlogopite from Mt. Vulture (Potenza, Italy): An in situ X-ray single-crystal diffraction study. Am Miner 93:632–643
Weibye PC (1848) Beiträge zur topographischen Mineralogie Norwegens. Archiv für Mineralogie Geognosie Bergbau Hüttenkunde 22:465–544
Woodrow PJ (1967) The crystal structure of astrophyllite. Acta Cryst 22:673–678
Yakovenchuk V, Ivanyuk G, Pakhomovsky Ya, Men’shikov Yu (2005) Khibiny. Laplandia Minerals, Apatity
Yefimov AF, Dusmatov VD, Ganzeyev AA, Katayeva ZT (1971) Cesium kupletskite, a new mineral. Dokl Akad Nauk SSSR 197:140–143 (in Russian)
Zema M, Ventruti G, Lacalamita M, Scordari F (2010) Kinetics of Fe-oxidation/deprotonation process in Fe-rich phlogopite under isothermal conditions. Am Miner 95:1458–1466
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (Grant 14-05-31229) and the President of Russian Federation Grant for Young Candidates of Sciences (Grant MK-3296.2015.5). The XRD studies were done at the X-ray Diffraction and Geomodel Centers of St. Petersburg State University. Mössbauer facilities, FCH and YAA were supported by an NSERC Discovery Grant and Canada Foundation for Innovation Grants to FCH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhitova, E.S., Krivovichev, S.V., Hawthorne, F.C. et al. High-temperature behaviour of astrophyllite, K2NaFe7 2+Ti2(Si4O12)2O2(OH)4F: a combined X-ray diffraction and Mössbauer spectroscopic study. Phys Chem Minerals 44, 595–613 (2017). https://doi.org/10.1007/s00269-017-0886-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-017-0886-1