Abstract
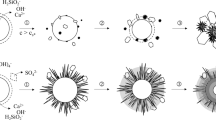
The phase transformation of schwertmannite, an iron oxyhydroxide sulfate nanomineral synthesized at room temperature and at 75 °C using H2O2 to drive the precipitation of schwertmannite from ferrous sulfate (Regenspurg et al. in Geochim Cosmochim Acta 68:1185–1197, 2004), was studied using high-resolution transmission electron microscopy. The results of this study suggest that schwertmannite synthesized using this method should not be described as a single phase with a repeating unit cell, but as a polyphasic nanomineral with crystalline areas spanning less than a few nanometers in diameter, within a characteristic ‘pin-cushion’-like amorphous matrix. The difference in synthesis temperature affected the density of the needles on the schwertmannite surface. The needles on the higher-temperature schwertmannite displayed a dendritic morphology, whereas the needles on the room-temperature schwertmannite were more closely packed. Visible lattice fringes in the schwertmannite samples are consistent with the powder X-ray diffraction (XRD) pattern taken on the bulk schwertmannite and also matched d-spacings for goethite, indicating a close structural relationship between schwertmannite and goethite. The incomplete transformation from schwertmannite to goethite over 24 h at 75 °C was tracked using XRD and TEM. TEM images suggest that the sample collected after 24 h consists of aggregates of goethite nanocrystals. Comparing the synthetic schwertmannite in this study to a study on schwertmannite produced at 85 °C, which used ferric sulfate, reveals that synthesis conditions can result in significant differences in needle crystal structure. The bulk powder XRD patterns for the schwertmannite produced using these two samples were indistinguishable from one another. Future studies using synthetic schwertmannite should account for these differences when determining schwertmannite’s structure, reactivity, and capacity to take up elements like arsenic. The schwertmannite synthesized by the Regenspurg et al. method produces a mineral that is consistent with the structure and morphology of natural schwertmannite observed in our previous study using XRD and TEM, making this an ideal synthetic method for laboratory-based mineralogical and geochemical studies that intend to be environmentally relevant.








Similar content being viewed by others
References
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11(7):36–42
Acero P, Ayora C, Torrento C, Nieto JM (2006) The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim Cosmochim Acta 70(16):4130–4139
Anschutz AJ, Penn RL (2005) Reduction of crystalline iron(III) oxyhydroxides using hydroquinone: influence of phase and particle size. Geochem Trans 6(3):60–66
Asta MP, Ayora C, Roman-Ross G, Cama J, Acero P, Gault AG, Charnock JM, Bardelli F (2010) Natural attenuation of arsenic in the Tinto Santa Rosa acid stream (Iberian Pyritic Belt, SW Spain): the role of iron precipitates. Chem Geol 271(1–2):1–12. doi:10.1016/j.chemgeo.2009.12.005
Banfield JF, Welch SA, Zhang HZ, Ebert TT, Penn RL (2000) Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 289(5480):751–754
Barham RJ (1997) Schwertmannite: a unique mineral, contains a replaceable ligand, transforms to jarosites, hematites, and/or basic iron sulfate. J Mater Res 12(10):2751–2758
Bigham JM, Nordstrom DK (2000) Iron and aluminum hydroxysulfates from acid sulfate waters. In: Alpers CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals-crystallography, geochemistry and environmental significance, vol 40, Reviews in mineralogy and geochemistryMineralogical Society of America, Washington, pp 351–403
Bigham JM, Schwertmann U, Carlson L, Murad E (1990) A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid-mine waters. Geochim Cosmochim Acta 54(10):2743–2758
Bigham JM, Carlson L, Murad E (1994) Schwertmannite, a new iron oxyhydroxysulphate from Pyhasalmi, Finland, and other localities. Mineral Mag 58(393):641–648
Bigham JM, Schwertmann U, Pfab G (1996a) Influence of pH on mineral speciation in a bioreactor simulating acid mine drainage. Appl Geochem 11(6):845–849
Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M (1996b) Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim Cosmochim Acta 60(12):2111–2121
Burleson DJ, Penn RL (2006) Two-step growth of goethite from ferrihydrite. Langmuir 22(1):402–409
Burton ED, Johnston SG (2012) Impact of silica on the reductive transformation of schwertmannite and the mobilization of arsenic. Geochimica Et Cosmochimica Acta 96:134–153
Burton ED, Bush RT, Sullivan LA, Mitchell DRG (2007) Reductive transformation of iron and sulfur in schwertmannite-rich accumulations associated with acidified coastal lowlands. Geochim Cosmochim Acta 71(18):4456–4473
Burton ED, Bush RT, Sullivan LA, Mitchell DRG (2008) Schwertmannite transformation to goethite via the Fe(II) pathway: reaction rates and implications for iron-sulfide formation. Geochim Cosmochim Acta 72(18):4551–4564. doi:10.1016/j.gca.2008.06.019
Burton ED, Johnston SG, Watling K, Bush RT, Keene AF, Sullivan LA (2010) Arsenic effects and behavior in association with the Fe(II)-catalyzed transformation of schwertmannite. Environ Sci Technol 44(6):2016–2021. doi:10.1021/es903424h
Bush R, Burton E, Sullivan L (2007) Catalytic action of aqueous Fe(II) and S(II) on the transformation of schwertmannite to goethite. Geochim Cosmochim Acta 71(15):A137–A137
Carlson L, Bigham JM, Schwertmann U, Kyek A, Wagner F (2002) Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ Sci Technol 36(8):1712–1719
Claassen JO, Meyer EHO, Rennie J, Sandenbergh RF (2002) Iron precipitation from zinc-rich solutions: defining the Zincor Process. Hydrometallurgy 67(1–3):87–108
Cornell RM, Schwertmann U (2003) The iron oxides, 2nd edn. Wiley-VCH, Weinheim
Davidson LE, Shaw S, Benning LG (2008) The kinetics and mechanisms of schwertmannite transformation to goethite and hematite under alkaline conditions. Am Mineral 93(8–9):1326–1337. doi:10.2138/am2008.2761
Echigo T, Aruguetea DM, Murayama M, Hochella MF (2012) Influence of size, morphology, surface structure, and aggregation state on reductive dissolution of hematite nanoparticles with ascorbic acid. Geochimica Et Cosmochimica Acta 90:149–162
Espana JS, Pamo EL, Santofimia E, Aduvire O, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20(7):1320–1356. doi:10.1016/j.apgeochem.2005.01.011
Fernandez-Martinez A, Timon V, Roman-Ross G, Cuello GJ, Daniels JE, Ayora C (2010) The structure of schwertmannite, a nanocrystalline iron oxyhydroxysulfate. Am Mineral 95:1312–1322
French RA, Caraballo MA, Kim B, Rimstidt J, Murayama M, Hochella MF (2012) The enigmatic iron oxyhydroxysulfate nanomineral schwertmannite: morphology, structure, and composition. Am Mineral 97(8–9):1469–1482
Hochella MF, Banfield JF (1995) Chemical weathering of silicates in nature: a microscopic perspective with theoretical considerations. In: White AF, Brantley SL (eds) Chemical weathering rates of silicate minerals, vol 31., vol 31The Mineralogical Society of America, Washington, pp 353–406
Hochella MF, Lower SK, Maurice PA, Penn RL, Sahai N, Sparks DL, Twining BS (2008) Nanominerals, mineral nanoparticles, and Earth systems. Science 319(5870):1631–1635
Hockridge JG, Jones F, Loan M, Richmond WR (2009) An electron microscopy study of the crystal growth of schwertmannite needles through oriented aggregation of goethite nanocrystals. J Cryst Growth 311(15):3876–3882. doi:10.1016/j.jcrysgro.2009.06.023
Janney DE, Cowley JM, Buseck PR (2000) Structure of synthetic 2-line ferrihydrite by electron nanodiffraction. Am Mineral 85(9):1180–1187
Jonsson J, Lovgren L (2006) Precipitation of secondary Fe(III) minerals from acid mine drainage. Appl Geochem 21(3):437–445
Jonsson J, Persson P, Sjoberg S, Lovgren L (2005) Schwertmannite precipitated from acid mine drainage: phase transformation, sulphate release and surface properties. Appl Geochem 20(1):179–191
Knorr KH, Blodau C (2007) Controls on schwertmannite transformation rates and products. Appl Geochem 22(9):2006–2015
Li D, Nielsen MH, Lee JRI, Frandsen C, Banfield JF, De Yoreo JJ (2012) Direction-specific interactions control crystal growth by oriented attachment. Science 336:1014–1018
Liu J, Aruguete DA, Jinschek JR, Rimstidt JD, Hochella MF (2008) The non-oxidative dissolution of galena nanocrystals: insights into mineral dissolution rates as a function of grain size, shape, and aggregation state. Geochim Cosmochim Acta 72(24):5984–5996. doi:10.1016/j.gca.2008.10.010
Liu J, Aruguete DM, Murayama M, Hochella MF (2009) Influence of size and aggregation on the reactivity of an environmentally and industrially relevant manomaterial (PbS). Environ Sci Technol 43(21):8178–8183. doi:10.1021/es902121r
Loan M, Cowley JM, Hart R, Parkinson GM (2004) Evidence on the structure of synthetic schwertmannite. Am Mineral 89(11–12):1735–1742
Madden AS, Hochella MF (2005) A test of geochemical reactivity as a function of mineral size: manganese oxidation promoted by hematite nanoparticles. Geochim Cosmochim Acta 69(2):389–398
Madden AS, Hochella MF, Luxton TP (2006) Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim Cosmochim Acta 70(16):4095–4104. doi:10.1016/j.gca.2006.06.1366
Maillot F, Morin G, Juillot F, Bruneel O, Casiot C, Ona-Nguema G, Wang Y, Lebrun S, Aubry E, Vlaic G (2012) Structure and reactivity of as (III)-and As (V)-rich schwertmannites and amorphous ferric arsenate sulfate from the Carnoulès Acid Mine Drainage. Comparison with biotic and abiotic model compounds and implications for As remediation. Geochimica Et Cosmochimica Acta, France
Majzlan J, Myneni SCB (2005) Speciation of iron and sulfate in acid waters: aqueous clusters to mineral precipitates. Environ Sci Technol 39(1):188–194
Michel FM, Ehm L, Antao SM, Lee PL, Chupas PJ, Liu G, Strongin DR, Schoonen MAA, Phillips BL, Parise JB (2007) The structure of ferrihydrite, a nanocrystalline material. Science 316(5832):1726–1729. doi:10.1126/science.1142525
Navrotsky A (2004) Energetic clues to pathways to biomineralization: precursors, clusters, and nanoparticles. Proc Natl Acad Sci USA 101(33):12096–12101
Penn RL, Erbs JJ, Gulliver DM (2006) Controlled growth of alpha-FeOOH nanorods by exploiting-oriented aggregation. J Cryst Growth 293(1):1–4. doi:10.1016/j.jcrysgro.2006.05.005
Regenspurg S, Peiffer S (2005) Arsenate and chromate incorporation in schwertmannite. Appl Geochem 20(6):1226–1239
Regenspurg S, Brand A, Peiffer S (2004) Formation and stability of schwertmannite in acidic mining lakes. Geochim Cosmochim Acta 68(6):1185–1197
Schroth AW, Parnell RA (2005) Trace metal retention through the schwertmannite to goethite transformation as observed in a field setting, Alta Mine, MT. Appl Geochem 20(5):907–917. doi:10.1016/j.apgeochem.2004.09.020
Schwertmann U (2000) Iron oxides in the laboratory: preparation and characterization, 2nd edn. Wiley-VCH, Weinheim, New York
Schwertmann U, Carlson L (2005) The pH-dependent transformation of schwertmannite to goethite at 25 °C. Clay Miner 40(1):63–66
Schwertmann U, Cornell RM (1991) Iron oxides in the laboratory: preparation and characterization. Wiley-VCH, New York
Waychunas GA (2001) Structure, aggregation and characterization of nanoparticles. In: Banfield JF, Navrotsky A (eds) Nanoparticles and the environment, vol 44., Reviews in mineralogy and geochemistry, vol 44The Mineralogical Society of America, Washington, pp 105–166
Wu CH, Reynolds WT Jr, Murayama M (2012) A software tool for automatic analysis of selected area diffraction patterns within Digital Micrograph™. Ultramicroscopy 112:10–14
Zinck JM, Dutrizac JE (1998) Environment—The behaviour of zinc, cadmium, thallium, tin and selenium during ferrihydrite precipitation from sulphate media. CIM Bull 91(1019):94–101
Acknowledgments
We thank the ICTAS Nanocharacterization and Fabrication Laboratory (NCFL) at Virginia Tech and the Materials Science and Engineering Nanoscale Materials Characterization Facility (MSE-NMCF) at the University of Virginia for the use of their Titan microscopes. Grants from the US Department of Energy (DE-FG02-06ER15786) and the Institute for Critical Technology and Applied Sciences at Virginia Tech provided major financial support for this project. We are also appreciative of the support from the National Science Foundation (NSF) and the Environmental Protection Agency through the Center for Environmental Implications of NanoTechnology (CEINT) funded under NSF Cooperative Agreement EF-0830093. Fellowship support for this research was provided by the National Science Foundation (NSF IGERT grant DGE-0504196). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of NSF, EPA, or DOE.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
French, R.A., Monsegue, N., Murayama, M. et al. The structure and transformation of the nanomineral schwertmannite: a synthetic analog representative of field samples. Phys Chem Minerals 41, 237–246 (2014). https://doi.org/10.1007/s00269-013-0641-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-013-0641-1