Abstract
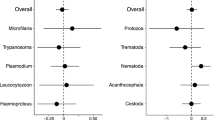
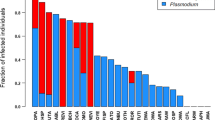
Hamilton and Zuk (Science 218:384–387, 1982) supported their influential hypothesis of parasite-mediated sexual selection based on a positive interspecific correlation between the prevalence of blood parasites and the expression of male displays in birds. However, subsequent studies provided mixed support for this relationship after considering several confounding factors. Here, we revisit this fundamental prediction by refining the analyses through implementation of recent methodological advancements. First, we distinguish between prevalence data obtained through microscopic and molecular tools, as PCR-based detection methods may be more sensitive for detecting infection. Second, we use quantitative estimates of both acoustic and visual signals of males, in which color measurements adopt the perspective of avian vision. Third, applying modern phylogenetic comparative approaches, we correct for phylogenetic inertia as well as heterogeneity in sampling effort. Fourth, we distinguish between prevalence transition states, as we compare species with and without evidence of infection and also monitor changes in parasite prevalence only in species in which blood parasites are detected. We show that given the considerable variation among populations, the repeatability of prevalence at the within-species level is modest. We failed to detect a strong interspecific relationship between the prevalence of blood parasites and sexual traits. However, we found that an evolutionary increase from zero to non-zero prevalence is likely to be accompanied by an increase in trait expression in males, but further increase from non-zero prevalence to a higher level of infection tends to be associated with a reduced degree of trait elaboration. Our results provide some support to the Hamilton and Zuk hypothesis, but the relationship between blood parasites and male displays varies among traits depending on degree of infection.


Similar content being viewed by others
References
Abouheif E (1999) A method for testing the assumption of phylogenetic independence in comparative data. Evol Ecol Res 1:895–909
Andersson S, Örnborg J, Andersson M (1998) Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc R Soc Lond B Biol Sci 265:445–450
Armenta JK, Dunn PO, Whittingham LA (2008) Quantifying avian sexual dichromatism: a comparison of methods. J Exp Biol 211:2423–2430
Arriero E, Møller AP (2008) Host ecology and life-history traits associated with blood parasite species richness in birds. J Evol Biol 21:1504–1513
Atkinson CT, Dusek RJ, Woods KL, Iko WM (2000) Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J Wildl Dis 36:197–204
Bennett PM, Owens IPF (2002) Evolutionary ecology of birds. Oxford University Press, Oxford
Bennett ATD, Cuthill IC, Norris K (1994) Sexual selection and the mismeasure of color. Am Nat 144:848–860
Bowmaker JK, Heath LA, Wilkie SE, Hunt DM (1997) Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vis Res 37:2183–2194
Buchanan KL, Catchpole CK, Lewis JW, Lodge A (1999) Song as an indicator of parasitism in the sedge warbler. Anim Behav 57:307–314
Byers BE, Kroodsma DE (2009) Female mate choice and songbird song repertoires. Anim Behav 77:13–22
Clayton DH, Pruett-Jones SG, Lande R (1992) Reappraisal of the interspecific prediction of parasite-mediated sexual selection: opportunity knocks. J Theor Biol 157:95–108
Cohen J (1988) Statistical power analysis for the behavioural sciences, 2nd edn. Lawrence Erlbaum, Hillsdale
Cosgrove CL, Day KP, Sheldon BC (2006) Coamplification of Leucocytozoon by PCR diagnostic tests for avian malaria: a cautionary note. J Parasitol 92:1362–1365
Cox FEG (1989) Parasites and sexual selection. Nature 341:289
Davis KE (2008) Reweaving the tapestry: a supertree of birds. Division of Environmental and Evolutionary Biology, University of Glasgow, Glasgow
Eaton MD (2005) Human vision fails to distinguish widespread sexual dichromatism among sexually “monochromatic” birds. Proc Natl Acad Sci USA 102:10942–10946
Endler JA, Lyles AM (1989) Bright ideas about parasites. Trends Ecol Evol 4:246–248
Evans KL, Gaston KJ, Sharp SP, McGowan A, Simeoni M, Hatchwell BJ (2009) Effects of urbanisation on disease prevalence and age structure in blackbird Turdus merula populations. Oikos 118:774–782
Fallon SM, Ricklefs RE (2008) Parasitemia in PCR-detected Plasmodium and Haemoproteus infections in birds. J Avian Biol 39:514–522
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Felsenstein J (2004) Inferring phylogenies. Sinauer Associates, Sunderland
Felsenstein J (2008) Comparative methods with sampling error and within-species variation: Contrasts revisited and revised. Am Nat 171:713–725
Freckleton RP (2009) The seven deadly sins of comparative analysis. J Evol Biol 22:1367–1375
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726
Freed LA, Cann RL (2003) On polymerase chain reaction tests for estimating prevalence of malaria in birds. J Parasitol 89:1261–1264
Freed LA, Cann RL (2006) DNA quality and accuracy of avian malaria PCR diagnostics: a review. Condor 108:459–473
Garamszegi LZ (2006) Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behav Ecol 17:682–687
Garamszegi LZ (2010) The sensitivity of microscopy and PCR-based detection methods affecting estimates of prevalence of blood parasites in birds. J Parasitol 96:1197–1203
Garamszegi LZ (2011) Climate change increases the risk of malaria in birds. Glob Chang Biol 17:1751–1759
Garamszegi LZ, Møller AP (2010) Effects of sample size and intraspecific variation in phylogenetic comparative studies: a meta-analytic review. Biol Rev 85:797–805
Garamszegi LZ, Møller AP, Török J, Michl G, Péczely P, Richard M (2004) Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav Ecol 15:148–157
Garamszegi LZ, Biard C, Eens M, Møller AP, Saino N (2007a) Interspecific variation in egg testosterone levels: implications for the evolution of bird song. J Evol Biol 20:950–964
Garamszegi LZ, Erritzøe J, Møller AP (2007b) Feeding innovations and parasitism in birds. Biol J Linn Soc 90:441–455
Garland T, Bennett AF, Rezende EL (2005) Phylogenetic approaches in comparative physiology. J Exp Biol 208:3015–3035
Garvin MC, Remsen JV (1997) An alternative hypothesis for heavier parasite loads of brightly colored birds: exposure at the nest. Auk 114:179–191
Gittleman JL, Kot M (1990) Adaptation: statistics and a null model for estimating phylogenetic effects. Syst Zool 39:227–241
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites. Science 218:384–387
Hart NS, Vorobyev M (2005) Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J Comp Physiol A 191:381–392
Hart NS, Partridge JC, Cuthill IC, Bennett ATD (2000) Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J Comp Physiol Sens Neural Behav Physiol 186:375–387
Harvey PH (2000) Why and how phylogenetic relationships should be incorporated into studies of scaling. In: Brown JH, West GB (eds) Scaling in Biology. Oxford University Press, Oxford, pp 253–265
Håstad O, Victorsson J, Ödeen A (2005) Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc Natl Acad Sci USA 102:6391–6394
Hausmann F, Arnold KE, Marshall NJ, Owens IPF (2003) Ultraviolet signals in birds are special. Proc R Soc Lond B Biol Sci 270:61–67
Hoberg EP, Brooks DR, Siegel-Causey D (1997) Host-parasite co-speciation: history, principles and prospects. In: Clayton DH, Moore J (eds) Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 213–235
Hofmann CM, Cronin TW, Omland KE (2008a) Evolution of sexual dichromatism. 1. Convergent losses of elaborate female coloration in New World Orioles (Icterus spp.). Auk 125:778–789
Hofmann CM, Cronin TW, Omland KE (2008b) Evolution of sexual dichromatism. 2. Carotenoids and melanins contribute to sexual dichromatism in New World Orioles (Icterus Spp.). Auk 125:790–795
Ives AR, Midford PE, Garland T (2007) Within-species variation and measurement error in phylogenetic comparative methods. Syst Biol 56:252–270
Jarvi SI, Schultz JJ, Atkinson CT (2002) PCR diagnostics underestimate the prevalence of avian malaria (Plasmodium relictum) in experimentally-infected passerines. J Parasitol 88:153–158
John JL (1995) Haematozoan parasites, mating systems and colorful plumages in songbirds. Oikos 72:395–401
Johnson SG (1991) Effects of predation, parasites, and phylogeny on the evolution of bright coloration in North American male passerines. Evol Ecol 5:52–62
Jovani R, Tella JL (2006) Parasite prevalence and sample size: misconceptions and solutions. Trends Parasitol 22:214–218
Martins EP, Hansen TF (1997) Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149:646–667
McManus DP, Bowles J (1996) Molecular genetic approaches to parasite identification: their value in diagnostic parasitology and systematics. Int J Parasitol 26:687–704
Mendes L, Piersma T, Lecoq M, Spaans B, Ricklefs RE (2005) Disease-limited distributions? Contrasts in the prevalence of avian malaria in shorebird species using marine and freshwater habitats. Oikos 109:396–404
Milinski M (2001) Bill Hamilton, sexual selection, and parasites. Behav Ecol 12:264–266
Møller AP (1990) Parasites and sexual selection: current status of the Hamilton and Zuk hypothesis. J Evol Biol 3:319–328
Møller AP (2008) Flight distance and blood parasites in birds. Behav Ecol 19:1305–1313
Møller AP, Birkhead TR (1994) The evolution of plumage brightness in birds is related to extrapair paternity. Evolution 48:1089–1100
Møller AP, Jennions MD (2002) How much variance can be explained by ecologists and evolutionary biologists. Oecologia 132:492–500
Møller AP, Nielsen JT (2007) Malaria and risk of predation: a comparative study of birds. Ecology 88:871–881
Møller AP, Christe P, Lux E (1999) Parasitism, host immune function, and sexual selection. Q Rev Biol 74:3–20
Møller AP, Henry P-Y, Erritzøe J (2000) The evolution of song repertoires and immune defence in birds. Proc R Soc Lond B 267:165–169
Møller AP, Nielsen JT, Garamszegi LZ (2006) Song post exposure, song features and predation risk. Behav Ecol 17:155–163
Møller AP, Nielsen JT, Garamszegi LZ (2008) Risk taking by singing males. Behav Ecol 19:41–53
Møller AP, Garamszegi LZ, Peralta-Sánchez JM, Soler JJ (2011) Migratory divides and their consequences for dispersal, population size and parasite–host interactions. J Evol Biol 24:1744–1755
Montgomerie R (2006) Analyzing colours. In: Hill GE, McGraw KJ (eds) Bird coloration. Harvard University Press, Cambridge, MA, pp 90–147
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Poulin R, Marshall LJ, Spencer HG (2000) Genetic variation and prevalence of blood parasites do not correlate among bird species. J Zool London 252:381–388
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Read AF (1987) Comparative evidence supports the Hamilton and Zuk hypothesis on parasites and sexual selection. Nature 328:68–70
Read AF (1988) Sexual selection and the role of parasites. Trends Ecol Evol 3:97–102
Read AF, Harvey PH (1989) Reassesment of comparative evidence for Hamilton and Zuk theory on the evolution of secondary sexual characters. Nature 339:618–620
Read AF, Weary DM (1990) Sexual selection and the evolution of bird song: a test of the Hamilton–Zuk hypothesis. Behav Ecol Sociobiol 26:47–56
Read AF, Weary DM (1992) The evolution of bird song: comparative analyses. Philos Trans R Soc Lond Ser B 338:165–187
Richard FA, Sehgal RNM, Jones HI, Smith TB (2002) A comparative analysis of PCR-based detection methods for avian malaria. J Parasitol 88:819–822
Ricklefs RE, Swanson BL, Fallon SM, Martinez-Abrain A, Scheuerlein A, Gray J, Latta SC (2005) Community relationships of avian malaria parasites in Southern Missouri. Ecol Monogr 75:543–559
Rohlf FJ (2006) A comment on phylogenetic correction. Evolution 60:1509–1515
Saino N, Galeotti P, Sacchi R, Møller AP (1997) Song and immunological conditions in male barn swallows (Hirundo rustica). Behav Ecol 8:364–371
Scheuerlein A, Ricklefs RE (2004) Prevalence of blood parasites in European passeriform birds. Proc R Soc Lond B 271:1363–1370
Seddon N, Tobias JA, Eaton MD, Ödeen A (2010) Human vision can provide a valid proxy for avian perception of sexual dichchromatism. Auk 127:283–292
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Sokal RR, Rohlf FJ (1995) Biometry. W. H. Freeman & Co., New York
Soma M, Garamszegi LZ (2011) Rethinking birdsong evolution: meta-analysis of the relationship between song complexity and reproductive success. Behav Ecol 22:363–371
Tella JL (2002) The evolutionary transition to coloniality promotes higher blood parasitism in birds. J Evol Biol 15:32–41
Tella JL, Blanco G, Forero MG, Gajon A, Donazar JA, Hiraldo F (1999) Habitat, world geographic range, and embryonic development of hosts explain the prevalence of avian hematozoa at small spatial and phylogenetic scales. Proc Natl Acad Sci USA 96:1785–1789
Underhill LG, Kaleita-Summers B (1995) Blood parasites in bright birds: testing the Hamilton–Zuk hypothesis in sub-Saharan Africa with an improved statistical method. Ostrich 66:10–14
Valkiūnas G (2005) Avian malaria parasites and other Haemosporidia. CRC Press, Boca Raton
Valkiūnas G, Bensch S, Iezhova TA, Krizanauskiene A, Hellgren O, Bolshakov CV (2006) Nested cytochrome B polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol 92:418–422
Valkiūnas G, Iezhova TA, Krizanauskiene A, Palinauskas V, Sehgal RNM, Bensch S (2008) A comparative analysis of microscopy and RCR-based detection methods for blood parasites. J Parasitol 94:1395–1401
Vorobyev M (2003) Coloured oil droplets enhance colour discrimination. Proc R Soc Lond B Biol Sci 270:1255–1261
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Waldenström J, Bensch S, Hasselquist D, Östman O (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194
Weatherhead PJ, Metz KJ, Bennett GF, Irwin RE (1993) Parasite faunas, testosterone and secondary sexual traits in male red-winged blackbirds. Behav Ecol Sociobiol 33:13–23
Weiss JB (1995) DNA probes and PCR for diagnosis of parasitic infections. Clin Microbiol Rev 8:113–130
Yezerinac SM, Weatherhead PJ (1995) Plumage coloration, differential attraction of vectors and Hematozoa infections in birds. J Anim Ecol 64:528–537
Acknowledgments
During this study, LZG was supported by a “Ramon y Cajal” research grant from the Spanish National Research Council [Consejo Superior de Investigaciones Científicas (CSIC), Spain] and by the “Plan Nacional” program of the Spanish government (LZG, ref. no. CGL2009-10652 and CGL2009-09439).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Pruett-Jones
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Comparative data of birds used to test for the relationship between the expression of sexual characters and prevalence of four blood parasite genera. Sample sizes (number of birds screened and found infected) are given separately for microscopy- and PCR-based detection methods (XLS 96 kb)
Rights and permissions
About this article
Cite this article
Garamszegi, L.Z., Møller, A.P. The interspecific relationship between prevalence of blood parasites and sexual traits in birds when considering recent methodological advancements. Behav Ecol Sociobiol 66, 107–119 (2012). https://doi.org/10.1007/s00265-011-1259-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1259-2