Abstract
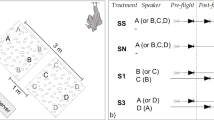
Eavesdropping on prey communication signals has never before been reported for a Palearctic bat species. In this study, we investigated whether lesser and greater mouse-eared bats, Myotis blythii oxygnathus and Myotis myotis, find tettigoniid bushcrickets (Tettigoniidae) by eavesdropping on their mate-attraction song. Tettigoniids are known to be the most important prey item for M. blythii oxygnathus, while carabid beetles and other epigaeic arthropods are the most important prey for its sibling species, M. myotis, in many places in Europe. M. myotis locates walking beetles by listening for their rustling sounds. We compared these two species’ response to four acoustic prey cues: calling song of two tettigoniid species, the rustling sound made by walking carabid beetles, and a control tone. Individuals of both bat species attacked the speaker playing tettigoniid song, which clearly indicates that both species eavesdrop on prey-generated advertisement signals. There were, however, species differences in response. M. blythii oxygnathus exhibited stronger predatory responses to the calling song of two species of tettigoniid than to the beetle rustling sound or the control. M. myotis, in contrast, exhibited stronger predatory responses to the beetle rustling and to one tettigoniid species but not the other tettigoniid or the control. Our study (1) for the first time demonstrates eavesdropping on prey communication signals for Palearctic bats and (2) gives preliminary evidence for sensory niche partitioning between these two sympatric sibling bat species.



Similar content being viewed by others
References
Anderson ME, Racey PA (1991) Feeding behavior of captive long-eared bats, Plecotus auritus. Anim Behav 42:489–493
Arak A, Eiriksson T (1992) Choice of singing sites by male bushcrickets (Tettigonia viridissima) in relation to signal propagation. Behav Ecol Sociobiol 30:365–372
Arlettaz R (1996) Feeding behaviour and foraging strategy of free-living mouse-eared bats, Myotis myotis and Myotis blythii. Anim Behav 51:1–11
Arlettaz R (1999) Habitat selection as a major resource partitioning mechanism between the two sympatric sibling bat species Myotis myotis and Myotis blythii. J Anim Ecol 68:460–471
Arlettaz R, Perrin N, Hausser J (1997) Trophic resource partitioning and competition between the two sibling bat species Myotis myotis and Myotis blythii. J Anim Ecol 66:897–911
Arlettaz R, Jones G, Racey PA (2001) Effect of acoustic clutter on prey detection by bats. Nature 414:742–745
Bailey WJ, Haythornthwaite S (1998) Risks of calling by the field cricket Teleogryllus oceanicus; potential predation by Australian long-eared bats. J Zool Lond 244:505–513
Belwood JJ, Morris GK (1987) Bat predation and its influence on calling behavior in neotropical katydids. Science 238:64–67
Bernays EA, Wcislo WT (1994) Sensory capabilities, information processing, and resource specialization. Q Rev Biol 69:187–204
Bogdanowicz W, van den Bussche R, Gajewska M, Postawa T, Harutyunyan M (2009) Ancient and contemporary DNA sheds light on the history of mouse-eared bats in Europe and the Caucasus. Acta Chiropterol 11:289–305
Bruns V, Burda H, Ryan MJ (1989) Ear morphology of the frog-eating bat (Trachops cirrhosus, family: Phyllostomidae): apparent specializations for low-frequency hearing. J Morphol 199:103–118
Buchler ER, Childs SB (1981) Orientation to distant sounds by foraging big brown bats (Eptesicus fuscus). Anim Behav 29:428–432
Fauna Europaea. www.faunaeur.org. Accessed 24 February 2010
Faure PA, Barclay RMR (1992) The sensory basis of prey detection by the long-eared bat, Myotis evotis, and the consequences for prey selection. Anim Behav 44:31–39
Faure PA, Hoy PP (2000) The sounds of silence: cessation of singing and song pausing are ultrasound-induced acoustic startle behaviors in the katydid Neoconocephalus ensiger (Orthoptera; Tettigoniidae). J Comp Physiol A 186:129–142
Fiedler J (1979) Prey catching with and without echolocation in the Indian False Vampire (Megaderma lyra). Behav Ecol Sociobiol 6:155–160
Goerlitz HR, Greif S, Siemers BM (2008) Cues for acoustic detection of prey: insect rustling sounds and the influence of walking substrate. J Exp Biol 211:2799–2806
Grossauer V (2010) Akustische Detektion der Echoortungssignale von Fledermäusen bei lautproduzierenden Laubheuschrecken. Masters thesis, University of Graz, Austria
Hartbauer M, Ofner E, Grossauer V, Siemers BM (2010) The cercal organ may provide singing tettigoniids a backup sensory system for the detection of eavesdropping bats. PLoS ONE, in press
Heller K-G (1988) Die Bioakustik der Europäischen Laubheuschrecken. J Margraf, Weikersheim
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Keuper A, Weidemann S, Kalmring K, Kaminski D (1988) Sound production and sound emission in seven species of European Tettigoniids. Part I. The different parameters of the song; their relation to the morphology of the bushcricket. Bioacoustics 1:31–48
Kolb A (1961) Sinnesleistungen einheimischer Fledermäuse bei der Nahrungssuche und Nahrungsauswahl auf dem Boden und in der Luft. Z Vergl Physiol 44:550–564
Lloyd JE, Wing SR (1983) Nocturnal aerial predation of fireflies by light-seeking fireflies. Science 222:634–635
Morris GK, Mason AC, Wall P, Belwood JJ (1994) High ultrasonic and tremulation signals in neotropical katydids (Orthoptera: Tettigoniide). J Zool 233:129–163
Neuhauser M (2004) Testing whether any of the significant tests within a table are indeed significant. Oikos 106:409–410
Nyberg DN (1971) Prey capture in the largemouth bass. Amer Midl Nat 86:128–144
Page RA, Ryan MJ (2005) Flexibility in assessment of prey cues: frog-eating bats and frog calls. Proc R Soc B 272:841–847
Peake TM (2005) Eavesdropping in communication networks. In: McGregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge, pp 13–37
Pereira MJR, Rebelo H, Rainho A, Palmeirim JM (2002) Prey selection by Myotis myotis (Vespertilionidae) in a Mediterranean region. Acta Chiropterol 4:183–193
Pianka ER (1981) Competition and niche theory. In: May R (ed) Theoretical ecology: principles and applications. Sinauer, Sunderland, pp 167–196
Pollak G, Henson OW Jr, Novick A (1972) Cochlear microphonic audiograms in the “pure tone” bat Chilonycteris parnellii parnellii. Science 176:66–68
Robert D, Amoroso J, Hoy RR (1992) The evolutionary convergence of hearing in a parasitoid fly and its cricket host. Science 258:1135–1137
Roberts SC, Gosling LM, Thornton EA, McClung J (2001) Scent marking by male mice under the risk of predation. Behav Ecol 12:698–670
Ruedi M, Mayer F (2001) Molecular systematics of bats in the genus Myotis (Vespertilionidae) suggests deterministic ecomorphological convergences. Mol Phylogenet Evol 21:436–448
Russo D, Jones G, Arlettaz R (2007) Echolocation and passive listening by foraging mouse-eared bats Myotis myotis and M. bythii. J Exp Biol 210:166–176
Ryan MJ, Tuttle MD (1987) The role of prey-generated sounds, vision, and echolocation in prey localization by the African bat Cardioderma cor (Megadermatidae). J Comp Physiol A 161:59–66
Ryan MJ, Tuttle MD, Rand AS (1982) Bat predation and sexual advertisement in a neotropical anuran. Am Nat 119:136–139
Safi K, Siemers BM (2010) Implications of sensory ecology for species coexistence: biased perception links predator diversity to prey size distribution. Evol Ecol 24:703–713
Schaub A, Ostwald J, Siemers BM (2008) Foraging bats avoid noise. J Exp Biol 211:3174–3180
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford
Schulze W, Schul J (2001) Ultrasound avoidance behaviour in the bushcricket Tettigonia viridissima (Orthoptera: Tettigoniidae). J Exp Biol 204:733–740
Siemers BM, Güttinger R (2006) Prey conspicuousness can explain apparent prey selectivity. Curr Biol 16:R157–R159
Siemers BM, Schnitzler HU (2004) Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429:657–661
Siemers BM, Swift SM (2006) Differences in sensory ecology contribute to resource partitioning in the bats Myotis bechsteinii and Myotis nattereri (Chiroptera: Vespertilionidae). Behav Ecol Sociobiol 59:373–380
Spangler HG (1984) Silence as a defense against predatory bats in two species of calling insects. Southwest Nat 29:481–488
Stowe MK, Turlings TCJ, Loughrin JH, Lewis WJ, Tumlinson JH (1995) The chemistry of eavesdropping, alarm, and deceit. Proc Natl Acad Sci USA 92:23–28
Swift SM, Racey PA (2002) Gleaning as a foraging strategy in Natterer’s bat Myotis nattereri. Behav Ecol Sociobiol 52:408–416
ter Hofstede HM, Ratcliffe JM, Fullard JH (2008) The effectiveness of katydid (Neoconocephalus ensiger) song cessation as antipredator defense against the gleaning bat Myotis septentionalis. Behav Ecol Sociobiol 63:217–226
Tilman D (2004) Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA 101:10854–10861
Tuttle MD, Ryan MJ (1981) Bat predation and the evolution of frog vocalizations in the Neotropics. Science 214:677–678
Tuttle MD, Ryan MJ, Belwood JJ (1985) Acoustical resource partitioning by two species of phyllostomid bats (Trachops cirrhosus and Tonatia sylvicola). Anim Behav 33:1369–1370
Zahn A, Rodrigues L, Rainho A, Paleirim JM (2007) Critical times of the year for Myotis myotis, a temperate zone bat: roles of climate and food resources. Acta Chiropterol 9:115–125
Zaykin DV, Zhivotovsky LA, Westfall PH, Weir BS (2002) Truncated product method for combining P values. Genet Epidemiol 22:170–185
Zuk M, Kolluru GR (1998) Exploitation of sexual signals by predators and parasitoids. Q Rev Biol 73:415–438
Acknowledgements
We are grateful to David Bethel, Ivailo Borissov, Tess Driessens, Stefan Greif, Antonia Hubancheva, Markus Schuller, and Sara Troxell for help and company during fieldwork, bat husbandry, and experiments. We thank Maike Schuchmann for assistance in amplitude measurements, Viktoria Grossauer for the evaluation of tettigoniid song parameters, and Leonie Baier for help with figure preparation. We thank the responsible Bulgarian authorities (MOEW-Sofia and RIOSV-Ruse) for granting us permission to conduct this research and the Directorate of the Rusenski Lom Nature Park (director Milko Belberov) as well as the Bulgarian Bat Research and Protection Group for cooperation and support. We are grateful to two anonymous reviewers for their insightful comments on the manuscript. This study was funded by the Max Planck Society (BMS), an Alexander von Humboldt Foundation Postdoctoral Research Fellowship (RAP), and a National Science Foundation Graduate Research Fellowship (PLJ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Jones
Electronic supplementary material
Below is the link to the electronic supplementary material.
Recording of a lesser mouse-eared bat, Myotis blythii oxygnathus, responding to playback of the calling song of the bushcricket species Tettigonia cantans. The speaker is in the center of the screen partially concealed by leaves. (MPG 4306 kb)
A different M. blythii oxygnathus individual responding to playback of the calling song of another bushcricket species, Tettigonia viridissima. (MPG 3658 kb)
Recording of a greater mouse-eared bat, Myotis myotis, responding to playback of the calling song of the bushcricket species Tettigonia cantans. (MPG 618 kb)
Rights and permissions
About this article
Cite this article
Jones, P.L., Page, R.A., Hartbauer, M. et al. Behavioral evidence for eavesdropping on prey song in two Palearctic sibling bat species. Behav Ecol Sociobiol 65, 333–340 (2011). https://doi.org/10.1007/s00265-010-1050-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1050-9