Abstract
Purpose
The dopamine D2 receptor (D2R) is important in the mediation of addiction. [123I]iodobenzamide (IBZM), a SPECT ligand for the D2R, has been used for in vivo studies of D2R availability in humans, monkeys, and rats. Although mouse models are important in the study of addiction, [123I]IBZM has not been used in mice SPECT studies. This study evaluates the use of [123I]IBZM for measuring D2R availability in mice.
Methods
Pharmacokinetics of [123I]IBZM in mice were studied with pinhole SPECT imaging after intravenous (i.v.) injection of [123I]IBZM (20, 40, and 70 MBq). In addition, the ability to measure the release of endogenous dopamine after amphetamine administration with [123I]IBZM SPECT was investigated. Thirdly, i.v. administration, the standard route of administration, and intraperitoneal (i.p.) administration of [123I]IBZM were compared.
Results
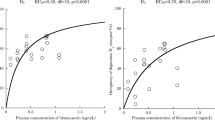
Specific binding of [123I]IBZM within the mouse striatum could be clearly visualized with SPECT. Peak specific striatal binding ratios were reached around 90 min post-injection. After amphetamine administration, the specific binding ratios of [123I]IBZM decreased significantly (−27.2%; n = 6; p = 0.046). Intravenous administration of [123I]IBZM led to significantly higher specific binding than i.p. administration of the same dose. However, we found that i.v. administration of a dose of 70 MBq [123I]IBZM might result in acute ethanol intoxication because ethanol is used as a preparative aid for the routine production of [123I]IBZM.
Conclusions
Imaging of D2R availability and endogenous dopamine release in mice is feasible using [123I]IBZM single pinhole SPECT. Using commercially produced [123I]IBZM, a dose of 40 MBq injected i.v. can be recommended.





Similar content being viewed by others
References
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 1988;85:5274–8.
Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 2002;5:169–74.
Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci 2006;9:1050–6.
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 2007;315:1267–70.
Nikolaus S, Beu M, Wirrwar A, Antke C, Muller HW. In vivo snapshots of the dopaminergic synapse in small animals. Mol Psychiatry 2005;10:516–8.
Nikolaus S, Larisch R, Wirrwar A, Jamdjeu-Noune M, Antke C, Beu M, et al. [123I]Iodobenzamide binding to the rat dopamine D2 receptor in competition with haloperidol and endogenous dopamine—an in vivo imaging study with a dedicated small animal SPECT. Eur J Nucl Med Mol Imaging 2005;32:1305–10.
Schiffer WK, Volkow ND, Fowler JS, Alexoff DL, Logan J, Dewey SL. Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse 2006;59:243–51.
Habraken JBA, de Bruin K, Shehata M, Booij J, Bennink R, van Eck Smit BLF, et al. Evaluation of high-resolution pinhole SPECT using a small rotating animal. J Nucl Med 2001;42:1863–9.
Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am 1984;1:612–9.
Andringa G, Drukarch B, Bol JGJM, de Bruin K, Sorman K, Habraken JBA, et al. Pinhole SPECT imaging of dopamine transporters correlates with dopamine transporter immunohistochemical analysis in the MPTP mouse model of Parkinson's disease. NeuroImage 2005;26:1150–8.
Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci 1991;11:2703–12.
Sun W, Ginovart N, Ko F, Seeman P, Kapur S. In vivo evidence for dopamine-mediated internalization of d2-receptors after amphetamine: differential findings with [3H]Raclopride versus [3H]Spiperone. Mol Pharmacol 2003;63:456–62.
Ginovart N. Imaging the dopamine system with in vivo [11C]raclopride displacement studies: understanding the true mechanism. Mol Imaging Biol 2005;7:45–52.
Beekman FJ, van der Have F, Vastenhouw B, van der Linden AJA, van Rijk PP, Burbach JPH, et al. U-SPECT-I: a novel system for submillimeter-resolution tomography with radiolabeled molecules in mice. J Nucl Med 2005;46:1194–200.
Vastenhouw B, van der Have F, van der Linden AJA, von Oerthel L, Booij J, Burbach JPH, et al. Movies of dopamine transporter occupancy with ultra-high resolution focusing pinhole SPECT. Mol Psychiatry 2007;12:984–7.
Hume SP, Gunn RN, Jones T. Pharmacological constraints associated with positron emission tomographic scanning of small laboratory animals. Eur J Nucl Med 1998;25:173–6.
Acton PD, Choi SR, Plossl K, Kung HF. Quantification of dopamine transporters in the mouse brain using ultra-high resolution single-photon emission tomography. Eur J Nucl Med Mol Imaging 2002;29:691–8.
Acton PD, Hou C, Kung MP, Plossl K, Keeney CL, Kung HF. Occupancy of dopamine D2 receptors in the mouse brain measured using ultra-high-resolution single-photon emission tomography and [123]IBF. Eur J Nucl Med Mol Imaging 2002;29:1507–15.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997;386:830–33.
Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 1997;386:827–30.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 2006;26:6583–8.
Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 2006;31:2716–27.
Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet 2003;116B:103–25.
Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci 2005;8:555–60.
Acknowledgments
We would like to thank Dr. Louk J.M.J. Vanderschuren for his critical reading of the manuscript and his useful comments.
The research was funded by a grant from the University Medical Center Utrecht and the Academic Medical Center.
The experiments comply with the laws of the Netherlands and were approved by the Committee for Animal Care of the Academic Medical Center. The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jongen, C., de Bruin, K., Beekman, F. et al. SPECT imaging of D2 dopamine receptors and endogenous dopamine release in mice. Eur J Nucl Med Mol Imaging 35, 1692–1698 (2008). https://doi.org/10.1007/s00259-008-0795-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0795-0