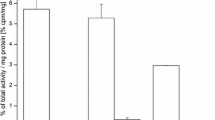
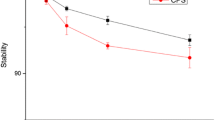
Abstract
Purpose
We have previously reported that 18F-FB-E[c(RGDyK)]2 (18F-FRGD2) allows quantitative PET imaging of integrin αvβ3 expression. However, the potential clinical translation was hampered by the relatively low radiochemical yield. The goal of this study was to improve the radiolabeling yield, without compromising the tumor targeting efficiency and in vivo kinetics, by incorporating a hydrophilic bifunctional mini-PEG spacer.
Methods
18F-FB-mini-PEG-E[c(RGDyK)]2 (18F-FPRGD2) was synthesized by coupling N-succinimidyl-4-18F-fluorobenzoate (18F-SFB) with NH2-mini-PEG-E[c(RGDyK)]2 (denoted as PRGD2). In vitro receptor binding affinity, metabolic stability, and integrin αvβ3 specificity of the new tracer 18F-FPRGD2 were assessed. The diagnostic value of 18F-FPRGD2 was evaluated in subcutaneous U87MG glioblastoma xenografted mice and in c-neu transgenic mice by quantitative microPET imaging studies.
Results
The decay-corrected radiochemical yield based on 18F-SFB was more than 60% with radiochemical purity of >99%. 18F-FPRGD2 had high receptor binding affinity, metabolic stability, and integrin αvβ3-specific tumor uptake in the U87MG glioma xenograft model comparable to those of 18F-FRGD2. The kidney uptake was appreciably lower for 18F-FPRGD2 compared with 18F-FRGD2 [2.0 ± 0.2%ID/g for 18F-FPRGD2 vs 3.0 ± 0.2%ID/g for 18F-FRGD2 at 1 h post injection (p.i.)]. The uptake in all the other organs except the urinary bladder was at background level. 18F-FPRGD2 also exhibited excellent tumor uptake in c-neu oncomice (3.6 ± 0.1%ID/g at 30 min p.i.).
Conclusion
Incorporation of a mini-PEG spacer significantly improved the overall radiolabeling yield of 18F-FPRGD2. 18F-FPRGD2 also had reduced renal uptake and similar tumor targeting efficacy as compared with 18F-FRGD2. Further testing and clinical translation of 18F-FPRGD2 are warranted.





Similar content being viewed by others
References
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673–87.
Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin αvβ3 antagonism. Anti-Cancer Agents Med Chem 2006;6:407–28.
Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science 1994;264:569–71.
Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer 2002;2:91–100.
Haubner R, Wester H-J, Weber WA, Mang C, Ziegler SI, Goodman SL, et al. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res 2001;61:1781–5.
Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, et al. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]galacto-RGD. PLoS Med 2005;2:e70.
Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, et al. Tumor targeting with radiolabeled αvβ3 integrin binding peptides in a nude mouse model. Cancer Res 2002;62:6146–51.
Chen X, Sievers E, Hou Y, Park R, Tohme M, Bart R, et al. Integrin αvβ3-targeted imaging of lung cancer. Neoplasia 2005;7:271–9.
Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. Micro-PET imaging of αvβ3-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging 2004;3:96–104.
Zhang X, Xiong Z, Wu X, Cai W, Tseng JR, Gambhir SS, et al. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J Nucl Med 2006;47:113–21.
Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, et al. MicroPET imaging of glioma αv-integrin expression using 64Cu-labeled tetrameric RGD eptide. J Nucl Med 2005;46:1707–18.
Cai W, Gambhir SS, Chen X. Multimodality tumor imaging targeting integrin αvβ3. Biotechniques 2005;39:S6–17.
Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide (18F-FBEM), and the synthesis of RGD peptide-based tracer for PET imaging of αvβ3 integrin expression. J Nucl Med 2006;47:1172–80.
Haubner R. αvβ3-integrin imaging: a new approach to characterise angiogenesis? Eur J Nucl Med Mol Imaging 2006;33 Suppl 11:54–63.
Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm 2006;3:472–87.
Ye Y, Bloch S, Xu B, Achilefu S. Design, synthesis, and evaluation of near infrared fluorescent multimeric RGD peptides for targeting tumors. J Med Chem 2006;49:2268–75.
Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chemistry 2003;9:2717–25.
Watson N, Duncan G, Annan WS, van der Walle CF. A tetravalent RGD ligand for integrin-mediated cell adhesion. J Pharm Pharmacol 2006;58:959–66.
Wester HJ, Kessler H. Molecular targeting with peptides or peptide-polymer conjugates: just a question of size? J Nucl Med 2005;46:1940–5.
Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003;2:214–21.
Chen X, Park R, Hou Y, Khankaldyyan V, Gonzales-Gomez I, Tohme M, et al. MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur J Nucl Med Mol Imaging 2004;31:1081–9.
Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I, et al. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor αvβ3-integrin expression. J Nucl Med 2004;45:1776–83.
Cai W, Olafsen T, Zhang X, Cao Q, Gambhir SS, Williams LE, et al. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti–carcinoembryonic antigen diabody. J Nucl Med 2007;48:304–10.
Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006;47:2048–56.
Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. In vitro and in vivo characterization of 64Cu-labeled Abegrin™, a humanized monoclonal antibody against integrin αvβ3. Cancer Res 2006;66:9673–81.
Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 1988;54:105–15.
Harris TD, Kalogeropoulos S, Nguyen T, Dwyer G, Edwards DS, Liu S, et al. Structure-activity relationships of 111In- and 99mTc-labeled quinolin-4-one peptidomimetics as ligands for the vitronectin receptor: potential tumor imaging agents. Bioconjug Chem 2006;17:1294–313.
Onthank DC, Liu S, Silva PJ, Barrett JA, Harris TD, Robinson SP, et al. 90Y and 111In complexes of a DOTA-conjugated integrin αvβ3 receptor antagonist: different but biologically equivalent. Bioconjug Chem 2004;15:235–41.
Harris TD, Kalogeropoulos S, Nguyen T, Liu S, Bartis J, Ellars C, et al. Design, synthesis, and evaluation of radiolabeled integrin αvβ3 receptor antagonists for tumor imaging and radiotherapy. Cancer Biother Radiopharm 2003;18:627–41.
Mousa SA, Mohamed S, Wexler EJ, Kerr JS. Antiangiogenesis and anticancer efficacy of TA138, a novel αvβ3 antagonist. Anticancer Res 2005;25:197–206.
Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, et al. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol 2004;31:179–89.
Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc 2004;126:5730–9.
Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, et al. Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med 2004;45:892–902.
Schottelius M, Poethko T, Herz M, Reubi JC, Kessler H, Schwaiger M, et al. First 18F-labeled tracer suitable for routine clinical imaging of sst receptor-expressing tumors using positron emission tomography. Clin Cancer Res 2004;10:3593–606.
Kilbourn MR, Dence CS, Welch MJ, Mathias CJ. Fluorine-18 labeling of proteins. J Nucl Med 1987;28:462–70.
Wester HJ, Hamacher K, Stoecklin G. A comparative study of n.c.a. fluorine-18 labeling of proteins via acylation and photochemical conjugation. Nucl Med Biol 1996;23:365–72.
Wilbur DS. Radiohalogenation of proteins: an overview of radionuclides, labeling methods and reagents for conjugate labeling. Bioconjug Chem 1992;3:432–70.
Marik J, Sutcliffe JL. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett 2006;47:6681–4.
Lee CC, Sui G, Elizarov A, Shu CJ, Shin YS, Dooley AN, et al. Multistep synthesis of a radiolabeled imaging probe using integrated microfluidics. Science 2005;310:1793–6.
Bach-Gansmo T, Danielsson R, Saracco A, Wilczek B, Bogsrud TV, Fangberget A, et al. Integrin receptor imaging of breast cancer: a proof-of-concept study to evaluate 99mTc-NC100692. J Nucl Med 2006;47:1434–9.
Acknowledgements
This work was supported in part by National Institute of Biomedical Imaging and Bioengineering (NIBIB) (R21 EB001785), National Cancer Institute (NCI) (R21 CA102123, P50 CA114747, U54 CA119367, and R24 CA93862), Department of Defense (DOD) (W81XWH-04-1-0697, W81XWH-06-1-0665, W81XWH-06-1-0042, and DAMD17-03-1-0143), and a Benedict Cassen Postdoctoral Fellowship from the Education and Research Foundation of the Society of Nuclear Medicine (to W. Cai). We thank Dr. David W. Dick from the cyclotron facility for 18F-F− production.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhanhong Wu and Zi-Bo Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Cell-binding assay of PRGD2, FPRGD2, RGD2, and FRGD2 using U87MG cells (integrin αvβ3-positive human glioblastoma) and 125I-echistatin as the radioligand. IC50 values for PRGD2, FPRGD2, RGD2, and FRGD2 were 70.1 ± 3.5, 40.6 ± 4.6, 26.1 ± 3.2, and 55.1 ± 6.5 nmol/l, respectively. Data points shown are means of triplicate samples (DOC 25 kb).
Rights and permissions
About this article
Cite this article
Wu, Z., Li, ZB., Cai, W. et al. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of αvβ3 integrin expression. Eur J Nucl Med Mol Imaging 34, 1823–1831 (2007). https://doi.org/10.1007/s00259-007-0427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0427-0