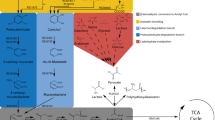
Abstract
Although economically efficient biomass conversion depends on the utilization of the complete cell wall (biorefinery concept), including polysaccharides and lignin, current biofuels research concentrate mostly on cellulose conversion, while lignin is viewed as a side-product that is used primarily as a thermal resource. Microbiological conversion of lignin is almost exclusive to fungi, usually resulting in increased cell mass and lignolytic enzymes. Some bacteria can also degrade lignin-related compounds using the β-ketoadipate pathway; for example, Rhodococcus opacus DSM 1069 can degrade coniferyl alcohol and grow on it as sole carbon source. Moreover, this strain belongs to the actinomycetes group that is also known for oleaginous species with lipid accumulation over 20%. Present work shows that R. opacus DSM 1069 and PD630 strains under nitrogen limiting conditions can convert lignin model compounds into triacylglycerols, also known as neutral lipids. 4-Hydroxybenzoic and vanillic acid lignin model compounds were used as sole carbon sources, and after brief adaptation periods, the cells not only began growing but accumulated lipids to the level of oleaginicity. These lipids were extracted for transesterification and analysis of fatty acid methyl esters showed good composition for biodiesel applications with no aromatics. Furthermore, the two strains showed distinct substrate metabolism and product profiles.



Similar content being viewed by others
References
Ahmad M, Roberts JN, Hardiman EM, Singh R, Eltis LD, Bugg TDH (2011) Identification of DypB from Rhodococcus jostii RHA1 as a lignin peroxidase. Biochemistry 50:5096–5107
Alvarez HM, Steinbüchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60:367–376
Alvarez HM, Mayer F, Fabritius D, Steinbüchel A (1996) Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch Microbiol 165:377–386
Alvarez HM, Kalscheuer R, Steinbüchel A (1997) Accumulation of storage lipids in species of Rhodococcus and Nocardia and effect of inhibitors and polyethylene glycol. Fett-Lipid 99:239–246
Alvarez HM, Kalscheuer R, Steinbüchel A (2000) Accumulation and mobilization of storage lipids by Rhodococcus opacus PD630 and Rhodococcus ruber NCIMB 40126. Appl Microbiol Biotechnol 54:218–223
Alvarez HM, Luftmann H, Silva RA, Cesari AC, Viale A, Wältermann M, Steinbüchel A (2002) Identification of phenyldecanoic acid as a constituent of triacylglycerols and wax ester produced by Rhodococcus opacus PD630. Microbiology 148:1407–1412
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Arabolaza A, Rodriguez E, Altabe S, Alvarez H, Gramajo H (2008) Multiple pathways for triacylglycerol biosynthesis in Streptomyces coelicolor. Appl Environ Microbiol 74:2573–2582
Bains J, Kaufman L, Farnell B, Boulanger MJ (2011) A product analog bound form of 3-oxoadipate-enol-lactonase (PcaD) reveals a multifunctional role for the divergent cap domain. J Mol Biol 406:649–658
Ben H, Ragauskas AJ (2011) NMR characterization of pyrolysis oils from Kraft lignin. Energy Fuel 25:2322–2332
Bleichrodt FS, Fischer R, Gerischer UC (2010) The β-ketoadipate pathway of Acinetobacter baylyi undergoes carbon catabolite repression, cross-regulation and vertical regulation, and is affected by Crc. Microbiology 156:1313–1322
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Brinkrolf K, Brune I, Tauch A (2006) Transcriptional regulation of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Genet Mol Res 5:773–789
Carrapiso AI, García C (2000) Development in lipid analysis: some new extraction techniques and in situ transesterification. Lipids 35:1167–1177
Chari RVJ, Whitman CP, Kozarich JW (1987) Absolute stereochemical course of muconolactone δ-isomerase and of 4-carboxymuconolactone decarboxylase: a 1H NMR “Ricochet” analysis. JACS 109:5520–5521
David K, Ragauskas AJ (2010) Switchgrass as an energy crop for biofuel production: a review of its lingo-cellulosic chemical properties. Energy Environ Sci 3:1182–1190
Davis JR, Sello JK (2010) Regulation of genes in Streptomyces bacteria required for catabolism of lignin-derived aromatic compounds. Appl Microbiol Biotechnol 86:921–929
Eggeling L, Sahm H (1980) Degradation of conyferyl alcohol and other lignin-related aromatic compounds by Nocardia sp. DSM 1069. Arch Microbiol 126:141–148
Eulberg D, Lakner S, Golovleva LA, Schlömann M (1998) Characterization of a protocatechuate catabolic gene cluster from Rhodococcus opacus 1CP: evidence for a merged enzyme with 4-carboxymuconolactone-decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity. J Bacteriol 180:1072–1081
Halak S, Lehtiö L, Basta T, Bürger S, Contzen M, Stolz A, Goldman A (2006) Structure and function of the 3-carboxy-cis, cis-muconate lactonizing enzyme from the protocatechuate degradative pathway of Agrobacterium radiobacter S2. FEBS J 273:5169–5182
Harris SP, Fujiwara N, Mealey RH, Alperin DC, Naka T, Goda R, Hines SA (2010) Identification of Rhodococcus equi lipids recognized by host cytotoxic T lymhocytes. Microbiology 156:1836–1847
Harwood CS, Parales RE (1996) The β-ketoadipate pathway and the biology of self identity. Annu Rev Microbiol 50:553–590
Jiménez JI, Miñambres B, García JL, Díaz E (2002) Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ Microbiol 4:824–841
Kadakol JC, Kamanavalli CM (2010) Biodegradation of eugenol by Bacillus Cereus strain PN24. E-J Chem 7:474–480
Kim SJ, Kweon O, Jones RC, Edmondson RD, Cerniglia CE (2008) Genomic analysis of polycyclic aromatic hydrocarbon degradation in Mycobacterium vanbaalenii PYR-1. Biodegradation 19:859–881
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuel 22:1358–1364
Kosa M, Ragauskas AJ (2011) Lipids from heterotrophic microbes: advances in metabolism research. Trends Biotechnol 29:53–61
Kosa M, Ben H, Theliander H, Ragauskas AJ (2011) Pyrolysis oils from CO2 precipitated Kraft lignin. Green Chem. doi:10.1039/C1GC15818J
Kurosawa K, Bocazzi P, Almeida NM, Sinskey AJ (2010) High-cell-density batch fermentation of Rhodococcus opacus PD630 using a high glucose concentration for triacylglycerol production. J Biotechnol 147:212–218
Lipscomb J (1992) Mechanistic aspects of dihydroxybenzoate dioxygenases. Met Ions Biol Syst 28:243–298
Nagy M, Kosa M, Theliander H, Ragauskas AJ (2010) Characterization of CO2 precipitated Kraft lignin to promote its utilization. Green Chem 12:31–34
Nichols NN, Harwood CS (1997) PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J Bacteriol 179:5056–5061
Pan X, Xie D, Yu WR, Lam D, Saddler JN (2007) Pretreatment of lodgepole pine killed by mountain pine beetle using the ethanol organosolv process: fractionation and process optimization. Ind Eng Chem Res 46:2609–2617
Parke D (1997) Acquisition, reorganization, and merger of genes: novel management of the β-ketoadipate pathway in Agrobacterium tumefaciens. FEMS Microbiol Lett 146:3–12
Patrauchan MA, Florizone C, Dosanjh M, Mohn WW, Davies J, Eltis LD (2005) Catabolism of benzoate and phthalate in Rhodococcus sp. Strain RHA1: redundancies and convergence. J Bacteriol 187:4050–4063
Pu Y, Zhang D, Singh PM, Ragauskas AJ (2008) The new forestry biofuels sector. Biofuels, Bioprod Biorefin 2:58–73
Pu Y, Kosa M, Kalluri UC, Tuskan GA, Ragauskas AJ (2011) Challenges of the utilization of wood polymers: how can they be overcome? Appl Microbiol Biotechnol 91:1525–1536
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward fro biofuels and biomaterials. Science 311:484–487
Ratledge C, Wynn JP (2002) The biochemistry and biotechnology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Sannigrahi P, Ragauskas AJ, Miller SJ (2010) Lignin structural modifications resulting from ethanol organosolv treatment of loblolly pine. Energy Fuel 24:683–689
Santala S, Efimova E, Kivinen V, Larjo A, Aho T, Karp M, Santala V (2011) Improved triacylglycerol production in Acinetobacter baylyi ADP1 by metabolic engineering. Microb Cell Fact 10:36
Schlegel HG, Kaltwasser H, Gottschalk G (1961) Ein submersverfahren zur kultur wasserstoffoxydierender bakterien: wachstumsphysiologische untersuchungen. Arch Microbiol 38:209–222
Singh P, Sulaiman O, Hashim R, Rupani PF, Peng LC (2010) Biopulping of lignocellulosic material using different fungal species: a review. Rev Environ Sci Biotechnol 9:141–151
Valley MP, Brown CK, Burk DL, Vetting MW, Ohlendorf DH, Lipscomb JD (2005) Roles of the equatorial tyrosyl iron ligand of protocatechuate 3,4- dioxygenase in catalysis. Biochem 44:11024–11039
Vetting MW, D’Argenio DA, Ornston LN, Ohlendorf DH (2000) Structure of Acinetobacter strain ADP1 protocatechuate 3,4-dioxygenase at 2.2 Å resolution: implications for the mechanism of an intradiol dioxygenase. Biochem 39:7943–7955
Voss I, Steinbüchel A (2001) High cell density cultivation of Rhodococcus opacus for lipid production at pilot-plant scale. Appl Microbiol Biotechnol 55:547–555
Warhurst AM, Fewson CA (1994) Biotransformations catalyzed by the genus Rhodococcus. Crit Rev Biotechnol 14:29–73
Widsten P, Kandelbauer A (2008) Laccase applications in the forest products industry: a review. Enzyme Microb Technol 42:293–307
Yang J, Wang Y, Woolridge EM, Arora V, Petsko GA, Kozarich JW, Ringe D (2004) Crystal structure of 3-carboxy-cis, cis-muconate lactonizing enzyme from pseudomonas putida, a fumarase class II type cycloisomerase: enzyme evolution in parallel pathways. Biochem 43:10424–10434
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599
Zhao KX, Huang Y, Chen X, Wang NX, Liu SJ (2010) PcaO positively regulates pcaHG of the β-ketoadipate pathway in Corynebacterium glutamicum. J Bacteriol 192:1565–1572
Zinoviev S, Mueller-Langer F, Das P, Bertero N, Fornasiero P, Kaltschmitt M, Centi G, Miertus S (2010) Next-generation biofuels: survey of emerging technologies and sustainability issues. ChemSusChem 3:1106–1133
Acknowledgements
The authors are grateful to the “Southern Pine Based Biorefinery Centre” (DOE award number DE-EE0003144) for financial support. M. K. is also thankful for the PSE (Paper Science and Engineering) program at Georgia Tech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kosa, M., Ragauskas, A.J. Bioconversion of lignin model compounds with oleaginous Rhodococci . Appl Microbiol Biotechnol 93, 891–900 (2012). https://doi.org/10.1007/s00253-011-3743-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3743-z