Abstract
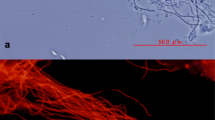
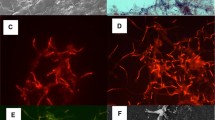
Primers targeting 16S rRNA genes were designed to detect and quantify Eikelboom type 021N organisms by real-time PCR. Eikelboom type 021N filamentous bulking was induced in a laboratory-scale sequencing batch reactor and the evolution of Eikelboom type 021N 16S rRNA and 16S rRNA genes was monitored. A significant correlation was found between the sludge volume index and the amount of these filamentous organisms present in the sludge (r 2=94.6%, n=10, P<0.01), as measured by real-time PCR. The amount of Eikelboom type 021N 16S rRNA genes increased by a factor of 21 during the experiment, while the 16S rRNA increased by a factor of 33. Moreover, Eikelboom type 021N 16S rRNA increased with increased feeding frequency. It was observed that the RNA:DNA ratio peaked before the sludge volume index increased. In parallel, a fluorescence in situ hybridization study indicated a factor of four increase in the length of Eikelboom type 021N filaments, due to a factor of two increase in both length and number of Eikelboom type 021N filaments. Further, an increase in the fraction of filaments extending outside the activated sludge flocs was observed (19–55%). Monitoring of 16S rRNA genes and 16S rRNA of Eikelboom type 021N was shown to be valuable in evaluating activated sludge settling characteristics; and measuring RNA:DNA ratios may be used as an early warning tool for sludge bulking.



Similar content being viewed by others
References
Amann RI (1995) In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes. In: Akkermans ADL, Elsas JD van, Bruijn FJ de (eds) Molecular microbial ecology manual. Kluwer, Dordrecht, pp 1–15
Amann RI, Wolfgang L (2000) Ribosomal RNA-targeted nucleic acid probes for studies in microbial ecology. FEMS Microb Rev 555–565
Amann RI, Krumholz L, Stahl DA (1990) Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 172:762–770
Biesterfeld S, Figueroa L, Hernandez M, Russell P (2001) Quantification of nitrifying bacterial populations in a full-scale trickling filter using fluorescent in situ hybridisation. Water Environ Res 73:329–338
Boon N, Goris J, De Vos P, Verstraete W, Top EM (2000) Bioaugmentation of activated sludge by an indigenous 3-chloroaniline degrading Comamonas testosteroni strain, I2 gfp. Appl Environ Microbiol 66:2906–2913
Boon N, Top EM, Verstraete W, Siciliano SD (2003) Bioaugmentation as a tool to protect the structure and function of an activated-sludge microbial community against a 3-chloroaniline shock load. Appl Environ Microbiol 69:1511–1520
Clauss F, Hélaine D, Balavoine C, Bidault A (1998) Improving activated sludge floc structure and aggregation for enhanced settling and thickening performances. Water Sci Technol 38:35–44
Clauss F, Balavoine C, Hélaine D, Martin G (1999) Controlling the settling of activated sludge in pulp and paper wastewater treatment plants. Water Sci Technol 40:223–229
Daims H, Bruhl A, Amann R, Schleifer KH, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
Daims H, Ramsing NB, Schleifer KH, Wagner M (2001) Cultivation-independent, semiautomatic determination of absolute bacterial cell numbers in environmental samples by fluorescence in situ hybridization. Appl Environ Microbiol 67:5810–5818
Eikelboom DH (2000) Process control of activated sludge plants by microscopic investigation. IWA, London
Eikelboom DH, Buijsen HJJ van (1999) Handbuch für die mikroskopische schlammuntersuchung. Broschiert, Hirthammer
Goethals P, Nicoletto L, Kersters I, Bossier P, Verstraete W (1997) Effect of reactive oxygen intermediates on microbial cells and communities: a model for the beneficial effect of Nutriflok 50 S on activated sludge. Proc Int Symp Environ Biotechnol 1997
Greenberg AE, Clesceri LS, Eaton AD (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, D.C.
Guyer W, Kappeler J (1992) Modelling population dynamics in activated sludge systems. Water Sci Technol 25:93–103
Harmsen HJM, Kengen HMP, Akkermans ADL, Stams AJM, De Vos WM (1996) Detection and localisation of synthropic propionate-oxidizing bacteria in granular sludge by in situ hybridisation using 16S rRNA-based oligonucleotide probes. Appl Environ Microbiol 62:1656–1663
Heid C, Stevens J, Livak K, Williams P (1996) Real time quantitative PCR. Genome Res 6:986–994
Hernandez M, Jenkins D, Beaman B (1994) Mass and viability estimations of Nocardia in activated sludge and anaerobic digesters using conventional stains and immunofluorescent methods. Water Sci Technol 29:249–259
Howarth R, Unz RF, Seviour EM, Seviour RJ, Blackall LL, Pickup RW, Jones JG, Yaguchi J, Head IM (1999) Phylogenetic relationships of filamentous sulfur bacteria (Thiothrix spp. and Eikelboom type 021N bacteria) isolated from wastewater-treatment plants and description of Thiothrix eikelboomii sp. nov., Thiothrix unzii sp. nov., Thiothrix fructosivorans sp. nov. and Thiothrix defluvii sp. Nov. Int J Syst Bacteriol 49:1817–1827
Hwang Y, Tanaka T (1998) Control of Microthrix parvicella foaming in activated sludge. Water Res 32:1678–1686
Jenkins D, Richard MG, Daigger GT (1993) Manual on the causes and control of activated sludge bulking and foaming, 2nd edn. Lewis, Chelsea, Mich.
Kanagawa T, Kamagata Y, Aruga S, Kohno T, Horn M, Wagner M (2000) Phylogenetic analysis of and oligonucleotide probe development for Eikelboom type 021N filamentous bacteria isolated from bulking activated sludge. Appl Environ Microbiol 66:5043–5052
Kohno T (1988) Morphology, physiology, and nutrition of a sulfur-oxidizing filamentous organism isolated from activated sludge. Water Sci Technol 20:241–247
Liao J, Lou I, De los Reyes FL III (2004) Relationship of species-specific filament levels to filamentous bulking in activated sludge. Appl Environ Microbiol 70:2420–2428
Liu JR, Burrell P, Seviour EM, Soddell JA, Blackall LL, Seviour RJ (2000) The filamentous bacterial morphotype Nostocoida limicola I contains at least two previously described genera in the low G+C gram positive bacteria. Syst Appl Microbiol 23:528–534
Martins AMP, Pagilla K, Heijnen JJ, Loosdrecht MCM van (2004) Filamentous bulking sludge—a critical review. Water Res 38:793–817
Molin S, Givskov M (1999) Application of molecular tools for in situ monitoring of bacterial growth activity. Environ Microbiol 1:383–391
Nielsen PH, Andeasen K, Wagner M, Blackall LL, Lemmer H, Seviour RJ (1998) Variety of type 021N in activated sludge as determined by in situ substrate uptake pattern and in situ hybridization with fluorescent rRNA targeted probes. Water Sci Technol 37:423–430
Oerther DB, De los Reyes FL III, De los Reyes MF, Raskin L (2001) Quantifying filamentous microorganisms in activated sludge before, during, and after an incident of foaming by oligonucleotide probe hybridizations and antibody staining. Water Res 35:3325–3336
Øvreas L, Forney L, Daae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic lake Saelevannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol 63:3367–3373
Palm JC, Jenkins D, Parker DS (1980) The relationship between organic loading, dissolved oxygen concentration and sludge settleability in the completely-mixed actrivated sludge process. J Water Pollut Control Fed 52:2484–2506
Panicker G, Myers ML, Bej AK (2004) Rapid detection of Vibrio vulnificus in shellfish and Gulf of Mexico water by real-time PCR. Appl Environ Microbiol 70:498–507
Séka MA, Cabooter S, Verstraete W (2001) A test for predicting propensity of activated sludge to acute filamentous bulking. Water Environ Res 73:237–242
Sezgin M, Jenkins D, Parker D (1978) A unified theory of filamentous activated sludge bulking. J Water Pollut Control Fed 50:362–381
Small J, Call DR, Brockman FJ, Straub TM, Chandler DP (2001) Direct detection of 16S rRNA in soil extracts by using oligonucleotide microarrays. Appl Environ Microbiol 67:4708–4716
SPSS Inc (1990) SPSS reference guide. SPSS, Englewoods Cliffs, N.Y.
Stahl DA, Flesher B, Mansfield HR, Montgomery L (1988) Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol 54:1079–1084
Stainsby FM, Soddel J, Seviour R, Upton J, Goodfellow M (2002) Dispelling the “Nocardia amarae” myth: a phylogenetic and phenotypic study of mycolic acid-containing actinomycetes isolated from activated sludge foam. Water Sci Technol 46:81–90
Vanderhasselt A, Verstraete W (1999) Short-term effects of additives on sludge sedimentation characteristics. Water Res 33:381–390
Van der Waarde JJ, Geurkink B, Henssen M, Heijnen G (1998) Detection of filamentous and nitrifying bacteria in activated sludge with 16S rRNA probes. Water Sci Technol 37:475–479
Vansever S, Bossier P, Vanderhasselt A, Beeckman M, Van der Zanden J, Weytjens D, Mingneau C, Verstraete W (1997) Improvement of activated sludge performance by the addition of Nutriflok 50 S. Water Res 31:366–371
Wagner M, Loy A (2002) Bacterial community composition and function in sewage treatment systems. Curr Opin Biotechnol 13:218–227
Wagner M, Amann R, Kaempeer P, Assmus B, Hartmann A, Hutzler P, Springer N, Schleifer KH (1994) Identification and in situ detection of Gram-negative filamentous bacteria in activated sludge. Syst Appl Microbiol 17:405–417
Wanner J (1994) Activated sludge bulking and foaming control. Technomic, Basel
Williams TM, Unz RF (1985) Isolation and characterization of filamentous bacteria present in bulking activated sludge. Appl Microbiol Biotechnol 22:273–282
Acknowledgements
This research was funded by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT–Vlaanderen). The authors thank Sylvie Seurinck, Roeland Grommen, and Tom Van De Wiele for critical comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vervaeren, H., De Wilde, K., Matthys, J. et al. Quantification of an Eikelboom type 021N bulking event with fluorescence in situ hybridization and real-time PCR. Appl Microbiol Biotechnol 68, 695–704 (2005). https://doi.org/10.1007/s00253-005-1963-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1963-9