Abstract
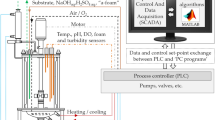
A growth-associated model was applied to the production of recombinant ovine interferon-τ (rOvIFN-τ) with Pichia pastoris for the purpose of manufacturing preclinical and clinical active material. This model predicts that product yields will be the greatest when the specific growth of the culture is maintained at a steady and optimal rate. However, rOvIFN-τ yields did not meet the expected linear model but most closely corresponded to a polynomial relationship. After transitioning from glycerol to methanol, product accumulated for 31–45 h, and then the yield decreased. This production shift, which has been termed decoupling, was clearly related to time on methanol and not culture density. It was determined that a correlation exists between the decoupling point and a drop in energy state of the cell when expressing β-galactosidase. By assigning decoupling as a constraint that limits productivity and by reformulating the growth medium, the time prior to decoupling increased to 46.8±2.4 h, product yield improved for rOvIFN-τ from 203 to 337 mg l−1, and the coefficient of variation for yield decreased from 67.9 to 23.3%. A robust and stable fermentation process was realized, resulting in a 210% improvement in total yield from 557±357 to 1,172±388 mg.






Similar content being viewed by others
References
Atkinson D, Walton G (1967) Adenosine triphosphate conservation in metabolic regulation: rate liver citrate cleavage enzyme. J Biol Chem 242:3239–3241
Babul J, Clifton D, Kretschmer M, Fraenkel D (1993) Glucose metabolism in Escherichia coli and the effect of increased amount of aldolase. Biochemistry 32:4685–4692
Brady C, Shimp R, Miles A, Whitmore M, Stowers A (2001) High-level production and purification of P20P2MSP119, an important vaccine antigen for malaria, expressed in the methylotrophic yeast Pichia pastoris. Protein Expr Purif 23:468–475
Byrne M, Titball R, Holley J, Smith L (2000) Fermentation, purification, and efficacy of a recombinant vaccine candidate against botulinum neurotoxin type F from Pichia pastoris. Protein Expr Purif 18:327–337
Chuang S-M, Chen L, Lambertson D, Anand M, Kinzy TG, Madura K (2005) Proteasome-mediated degradation of cotranslationally damaged proteins involves translation elongation factor 1A. Mol Cell Biol 25:403–413
Cregg JM, Barringer KJ, Hessler AY, Madden KR (1985) Pichia pastoris as a host system for transformations. Mol Cell Biol 5:3376–3385
Cregg JM, Tschopp J, Stillman C, Siegel R, Akong M, Craig W, Buckholz R, Madden K, Kellaris P, Davis G, Smiley B, Cruze J, Torregrossa R, Velice-lebi G, Thill G (1987) High-level expression and efficient assembly of hepatitis B surface antigen in the methylotrophic yeast, Pichia pastoris. Bio/technology 5:479–485
d’Anjou M, Daugulis J (2001) A rational approach to improving productivity in recombinant Pichia pastoris fermentation. Biotechnol Bioeng 72:1–11
Edwards K, Urban J, Schreiber G (1979) Relationship between protein synthesis and secretion in liver cells and the state of the adenine nucleotide system. Aust J Biol Sci 32:299–307
Files D, Ogawa M, Scaman C, Baldwin S (2001) A Pichia pastoris fermentation process for producing high-levels of recombinant human cystatin-C. Enzyme Microb Technol 29:335–340
Gleeson M, White C, Meininger D, Komives E (1998) Generation of protease-deficient strains and their use in heterologous protein expression. Methods Mol Biol 103:81–94
Jamieson J, Palade G (1968) Intracellular transport of secretory proteins in the pancreatic exocrine cell IV: metabolic requirements. J Cell Biol 39:589–603
Katakura Y, Zhang W, Guoqiang O, Takeshi K, Kishimoto M, Goto Y, Suga K-I (1998) Effect of methanol concentration on the production of human β2-glycoprotein I domain V by a recombinant Pichia pastoris: a simple system for the control of methanol concentration using a semiconductor gas sensor. J Ferment Bioeng 86:482–487
Kobayashi K, Kuwae S, Ohya T, Ohda T, Ohyama M, Tomomitsu K (2000) High level secretion of recombinant human serum albumin by fed-batch fermentation of the methylotrophic yeast, Pichia pastoris, based on optimal methanol feeding strategy. J Biosci Bioeng 90:280–288
Li Z, Xiong F, Lin Q, d’Anjou M, Daugulis AJ, Yang DS, Hew CL (2001) Low-temperature increases the yield of biologically active herring antifreeze protein in Pichia pastoris. Protein Expr Purif 21:438–445
Lin-Cereghino J, Geoff P, Ilgen C, Cregg JM (2002) Production of recombinant proteins in ferment or cultures of the yeast Pichia pastoris. Curr Opin Biotechnol 13:329–332
Miller J (1992) A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Woodbury, pp 72–74
Plantz B, Andersen J, Smith L, Meagher M, Schlegel V (2003) Detection of non-host viable contaminants in Pichia pastoris cultures and fermentation broths. J Ind Microbiol Biotech 30:643–650
Sinha J, Plantz B, Zhang W, Gouthro M, Schlegel V, Liu C-P, Meagher M (2003) Improved production of recombinant ovine interferon-τ by mut+ strain of Pichia pastoris using an optimized methanol feed profile. Biotechnol Prog 19:794–802
Sinha J, Plantz B, Inan M, Meagher M (2004) Causes of proteolytic degradation of secreted recombinant proteins production in methylotrophic yeast Pichia pastoris—case study with recombinant ovine interferon-τ. Biotechnol Bioeng 89:102–112
Stratton J, Chiruvolu V, Meagher M (1998) High cell-density fermentation. In: Higgins DR, Cregg JM (eds) Methods in molecular biology: Pichia protocols. Humana, Totowa, pp 107–120
Trentmann O, Khatri NK, Hoffmann F (2004) Reduced oxygen supply increases process stability and product yield with recombinant Pichia pastoris. Biotechnol Prog 20:1766–1775
Tschopp JF, Brust PF, Cregg JM, Stillman CA, Gingeras TR (1987) Expression of the lacZ gene from two methanol-regulated promoters in Pichia pastoris. Nucleic Acids Res 15:3859–3867
US Food and Drug Administration (2005) Code of federal regulations. General biological product standards, title 21, parts 610.9 and 610.13. US Government Printing Office, Washington, DC
Van Heeke G, Ott TL, Strauss A, Ammaturo D, Bazer FW (1996) High yield expression and secretion of the ovine pregnancy recognition hormone interferon-tau by Pichia pastoris. J Interferon Cytokine Res 16:119–126
Velkov VV, Matys VY, Sokolov DM (1999) How overproduction of foreign proteins affects physiology of the recombinant strains of Hansenula polymorpha. J Biosci 24:279–286
Wood T, Peretti S (1990) Depression of protein synthetic capacity due to cloned-gene expression in E. coli. Biotechnol Bioeng 36:865–877
Zhang W, Inan M, Meagher M (2000a) Fermentation strategies for recombinant protein expression in the methylotrophic yeast Pichia pastoris. Biotechnol Bioprocess Eng 5:275–287
Zhang W, Bevins M, Plantz B, Smith L, Meagher M (2000b) Modeling Pichia pastoris growth on methanol and optimizing the production of a recombinant protein, the heavy-chain fragment C of botulinum neurotoxin, serotype A. Biotechnol Bioeng 70:1–8
Zhang W, Smith L, Plantz B, Schlegel V, Meagher (2002) Design of methanol feed control in Pichia pastoris fermentations based upon a growth model. Biotechnol Prog 18:1392–1399
Zhang W, Hywood Potter KJ, Plantz BA, Schlegel VL, Smith LA, Meagher MM (2003) Pichia pastoris fermentation with mixed-feeds of glycerol and methanol: growth kinetics and production improvement. J Ind Microbiol Biotechnol 30:210–215
Acknowledgements
The funding for this research was generously supported by a grant provided by the Pepgen Corporation. We further acknowledge Dr. Gautam Sarath for data analysis, and the staff of the University of Nebraska Biological Process Development Facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plantz, B.A., Sinha, J., Villarete, L. et al. Pichia pastoris fermentation optimization: energy state and testing a growth-associated model. Appl Microbiol Biotechnol 72, 297–305 (2006). https://doi.org/10.1007/s00253-005-0271-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0271-8