Abstract
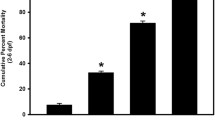
Selenium (Se) is an essential micronutrient that can be found at toxic concentrations in surface waters contaminated by runoff from agriculture and coal mining. Zebrafish (Danio rerio) embryos were exposed to aqueous Se in the form of selenate, selenite, and l-selenomethionine (SeMet) in an attempt to determine if oxidative stress plays a role in selenium embryo toxicity. Selenate and selenite exposure did not induce embryo deformities (lordosis and craniofacial malformation). l-selenomethionine, however, induced significantly higher deformity rates at 100 µg/L compared with controls. SeMet exposure induced a dose-dependent increase in the catalytic subunit of glutamate-cysteine ligase (gclc) and reached an 11.7-fold increase at 100 µg/L. SeMet exposure also reduced concentrations of TGSH, RGSH, and the TGSH:GSSG ratio. Pretreatment with 100 µM N-acetylcysteine significantly reduced deformities in the zebrafish embryos secondarily treated with 400 µg/L SeMet from approximately 50–10 % as well as rescued all three of the significant glutathione level differences seen with SeMet alone. Selenite exposure induced a 6.6-fold increase in expression of the glutathione-S-transferase pi class 2 (gstp2) gene, which is involved in xenobiotic transformation and possibly oxidative stress. These results suggest that aqueous exposure to SeMet can induce significant embryonic teratogenesis in zebrafish that are at least partially attributed to oxidative stress.








Similar content being viewed by others
References
Bergeron CM, Bodinof CM, Unrine JM, Hopkins WA (2010) Bioaccumulation and maternal transfer of mercury and selenium in amphibians. Environ Toxicol Chem 29:989–997. doi:10.1002/Etc.125
Bierl C, Voetsch B, Jin RC, Handy DE, Loscalzo J (2004) Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem 279:26839–26845. doi:10.1074/jbc.M401907200
Ceconi C, Curello S, Cargnoni A, Ferrari R, Albertini A, Visioli O (1988) The role of glutathione status in the protection against ischemic and reperfusion damage: effects of N-acetyl cysteine. J Mol Cell Cardiol 20:5–13. doi:10.1016/S0022-2828(88)80174-3
Deflora S, Bennicelli C, Camoirano A et al (1985) In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and or mutagenic compounds. Carcinogenesis 6:1735–1745
Di Giulio RT, Meyer JN (2008) Reactive oxygen species and oxidative stress. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. CRC Press, Boca Raton, pp 273–324
Eisler R (2000) Handbook of chemical risk assessment: health hazards to humans, plants, and animals. Lewis Publishers, Boca Raton
Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ (2009) Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 30:86–98. doi:10.1016/j.mam.2008.08.009
Franson JC, Hoffman DJ, Wells-Berlin A et al (2007) Effects of dietary selenium on tissue concentrations, pathology, oxidative stress, and immune function in common eiders (Somateria mollissima). J Toxicol Environ Health A 70:861–874. doi:10.1080/15287390701212760
Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML (2008) PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol Sci 106:193–205
Heinz GH, Hoffman DJ, Gold LG (1988) Toxicity of organic and inorganic selenium to mallard ducklings. Arch Environ Contam Toxicol 17:561–568
Hermes-Lima M, Storey KB (1996) Relationship between anoxia exposure and antioxidant status inthe frog Rana pipiens. Am J Physiol 217:918–925
Hilton JW, Hodson PV, Slinger SJ (1980) The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J Nutr 110:2527–2535
Hoffman DJ, Heinz GH (1998) Effects of mercury and selenium on glutathione metabolism and oxidative stress in Mallard ducks. Environ Toxicol Chem 17:161–166
Holm J, Palace V, Siwik P et al (2005) Developmental effects of bioaccumulated selenium in eggs and larvae of two salmonid species. Environ Toxicol Chem 24:2373–2381. doi:10.1897/04-402r1.1
Hussain SP, Amstad P, He PJ et al (2004) p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res 64:2350–2356. doi:10.1158/0008-5472.Can-2287-2
Ikeda H, Serria MS, Kakizaki I et al (2002) Activation of mouse Pi-class glutathione S-transferase gene by Nrf2(NF-E2-related factor 2) and androgen. Biochem J 364:563–570. doi:10.1042/BJ20011756
Ikeda H, Nishi S, Sakai M (2004) Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem J 380:515–521. doi:10.1042/BJ20031948
Janz DM, DeForest DK, Brooks ML et al (2010) Selenium toxicity to aquatic oganisms. In: Chapman PM, Adams JB, Brooks ML et al (eds) Ecological assessment of selenium in the aquatic environment. CRC Press, Boca Raton, pp 141–232
Jones DP, Eklow L, Thor H, Orrenius S (1981) Metabolism of hydrogen-peroxide in isolated hepatocytes: relative contributions of catalase and glutathione-peroxidase in decomposition of endogenously generated H2O2. Arch Biochem Biophys 210:505–516. doi:10.1016/0003-9861(81)90215-0
Jones PL, Kucera G, Gordon H, Boss JM (1995) Cloning and characterization of the murine manganous superoxide dismutase-encoding gene. Gene 153:155–161
Kroll KJ, Doroshov SI (1991) Vitellogenin: potential vehicle for selenium bioaccumulation in oocytes of the white sturgeon (Acipenser transmontanus). In: Williot P (ed) Acipenser. CEMAGREF, Bordeaux, pp 99–106
Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ (2004) Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys 423:116–125
Kupsco A, Schlenk D (2014) Mechanisms of selenomethionine developmental toxicity and the impacts of combined hypersaline conditions on Japanese medaka (Oryzias latipes). Environ Sci Technol 48:7062–7068. doi:10.1021/es5019948
Küster E, Altenburger R (2008) Oxygen decline in biotesting of environmental samples–Is there a need for consideration in the acute zebrafish embryo test? Environ Toxicol 23:745–750. doi:10.1002/tox.20377
Lavado R, Shi DL, Schlenk D (2012) Effects of salinity on the toxicity and biotransformation of l-selenomethionine in Japanese medaka (Oryzias latipes) embryos: mechanisms of oxidative stress. Aquat Toxicol 108:18–22. doi:10.1016/j.aquatox.2011.07.001
Lemly AD (2002) Selenium assessment in aquatic ecosystems: a guide for hazard evaluation and water quality criteria. Springer, New York
Lindberg TT, Bernhardt ES, Bier R et al (2011) Cumulative impacts of mountaintop mining on an Appalachian watershed. Proc Natl Acad Sci USA 108:20929–20934. doi:10.1073/pnas.1112381108
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Maher WA, Roach A, Doblin MA et al (2010) Environmental sources, speciation, and partitioning of selenium. In: Chapman PM, Adams WJ, Brooks ML et al (eds) Ecological assessment of selenium in the aquatic environment. CRC Press, Boca Raton, pp 47–92
Malek RL, Sajadi H, Abraham J, Grundy MA, Gerhard GS (2004) The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp Biochem Phys C 138:363–373
Massarsky A, Dupuis L, Taylor J, Eisa-Beygi S, Strek L, Trudeau VL, Moon TW (2013) Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 92:59–66
Miki K, Xu M, Gupta A, Ba Y, Tan Y, Al-Refaie W, Bouvet M, Makuuchi M, Moossa AR, Hoffman RM (2001) Methioninase cancer gene therapy with selenomethionine as suicide prodrug substrate. Cancer Res 61:6805–6810
Miller LL, Wang F, Palace VP, Hontela A (2007) Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol 83:263–271
Misra S, Niyogi S (2009) Selenite causes cytotoxicity in rainbow trout (Oncorhynchus mykiss) hepatocytes by inducing oxidative stress. Toxicol In Vitro 23:1249–1258
Misra S, Hamilton C, Niyogi S (2012) Induction of oxidative stress by selenomethionine in isolated hepatocytes of rainbow trout (Oncorhynchus mykiss). Toxicol In Vitro 26:621–629
Moldeus P, Cotgreave IA, Berggren M (1986) Lung protection by a thiol-containing antioxidant—N-acetylcysteine. Respiration 50:31–42
Muscatello JR, Bennett PM, Himbeault KT, Belknap AM, Janz DM (2006) Larval deformities associated with selenium accumulation in northern pike (Esox lucius) exposed to metal mining effluent. Environ Sci Technol 40:6506–6512
Niimi AJ, LaHam QN (1976) Relative toxicity of organic and inorganic compounds of selenium to newly hatched zebrafish (Brachydanio rerio). Can J Zool 54:501–509
Oberley LW, Oberley TD (1988) Role of antioxidant enzymes in cell Immortalization and transformation. Mol Cell Biochem 84:147–153. doi:10.1007/Bf00421049
Palace VP, Spallholz JE, Holm J, Wautier K, Evans RE, Baron CL (2004) Metabolism of selenomethionine by rainbow trout (Oncorhynchus mykiss) embryos can generate oxidative stress. Ecotoxicol Environ Saf 58:17–21. doi:10.1016/j.ecoenv.2003.08.019
Palmer MA, Bernhardt ES, Schlesinger WH, Eshleman KN, Foufoula-Georgiou E, Hendryx MS, Lemly AD, Likens GE, Loucks OL, Power ME, White PS, Wilcock PR (2010) Science and regulation. Mountaintop mining consequences. Science 327:148–149
Presser TS, Ohlendorf HM (1987) Biogeochemical cycling of selenium in the San-Joaquin Valley, California, USA. J Environ Manage 11:805–821. doi:10.1007/Bf01867247
Schlenk D, Zubcov N, Zubcov E (2003) Effects of salinity on the uptake, biotransformation, and toxicity of dietary seleno-l-methionine to rainbow trout. Toxicol Sci 75:309–313
Schlenk D, Celander M, Gallagher EP et al (2008) Biotransformation in fishes. In: Di Giulio R, Hinton DE (eds) The toxicology of fishes. CRC Press, Boca Raton, pp 153–234
Shilo S, Aharoni-Simon M, Tirosh O (2004) Selenium attenuates expression of MnSOD and Uncoupling Protein 2 in J774.2 macrophages: molecular mechanism for its cell-death and antiinflammatory activity. Antioxid Redox Signal 7:276–286
Shilo S, Pardo M, Aharoni-Simon M, Glibter S, Tirosh O (2008) Selenium supplementation increases liver MnSOD expression: molecular mechanism for hepato-protection. J Inorg Biochem 102:110–118. doi:10.1016/j.jinorgbio.2007.07.027
Spallholz JE (1997) Free radical generation by selenium compounds and their prooxidant toxicity. Biomed Environ Sci 10:260–270
Strecker R, Seiler TB, Hollert H, Braunbeck T (2011) Oxygen requirements of zebrafish (Danio rerio) embryos in embryos toxicity tests with environmental samples. Comp Biochem Physiol C 153:318–327. doi:10.1016/j.cbpc.2010
Suzuki T, Takagi Y, Osanai H, Li L, Takeuchi M, Katoh Y, Kobayashi M, Yamamoto M (2005) Pi class glutathione S-transferase genes are regulated by Nrf 2 through an evolutionarily conserved regulatory element in zebrafish. Biochem J 388:65–73
Teh SJ, Deng X, Deng DF et al (2004) Chronic effects of dietary selenium on juvenile Sacramento splittail (Pogonichthys macrolepidotus). Environ Sci Technol 38:6085–6093. doi:10.1021/Es049545+
Timme-Laragy AR, Van Tiem LA, Linney EA, Di Giulio RT (2009) Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol Sci 109:217–227. doi:10.1093/toxsci/kfp038
Unrine JM, Jackson BP, Hopkins WA, Romanek C (2006) Isolation and partial characterization of proteins involved in maternal transfer of selenium in the western fence lizard (Sceloporus occidentalis). Environ Toxicol Chem 25:1864–1867. doi:10.1897/05-598r.1
Useko CY, Harper SL, Tanguay RL (2009) Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol 229:44–55
Van Tiem LA, Di Giulio RT (2011) AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE- and ARE-associated gene induction in zebrafish (Danio rerio). Toxicol Appl Pharmacol 254:280–287
Vidal D, Bay SM, Schlenk D (2005) Effects of dietary selenomethionine on larval rainbow trout (Oncorhynchus mykiss). Arch Environ Contam Toxicol 49:71–75
Wang Z, Jiang C, Lu J (2002) Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog 34:113–120. doi:10.1002/mc.10056
Wild AC, Mulcahy RT (2000) Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic Res 32:281–301. doi:10.1080/10715760000300291
Acknowledgments
The authors thank Drs. D. Hinton, A. Massarsky and A. Bone, T. Lindberg, J. Brandt, and M. Chernick for assistance and advice. This work was made possible by a grant to The Duke University Nicholas School of the Environment from the Foundation for the Carolinas, as well as the National Institute of Environmental Health Science grant T32ES07031 to support the Duke Integrated Toxicology and Environmental Health Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arnold, M.C., Forte, J.E., Osterberg, J.S. et al. Antioxidant Rescue of Selenomethionine-Induced Teratogenesis in Zebrafish Embryos. Arch Environ Contam Toxicol 70, 311–320 (2016). https://doi.org/10.1007/s00244-015-0235-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-015-0235-7