Abstract
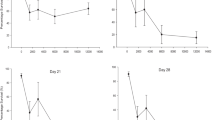
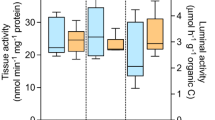
The kinetics of the bioaccumulation of malathion (O,O-dimethyl phosphorodithioate of diethyl mercaptosuccinate) and the biological impact of exposure for tiger salamanders, Ambystoma tigrinum, were assessed through exposure to soil surface contaminated with 50 μg/cm2 or 100 μg/cm2 malathion and ingestion of an earthworm exposed to soil contaminated with 200 μg/cm2 malathion. Malathion and malaoxon burdens in salamanders sampled at different times after exposure(s) were measured by gas chromatography in four tissue/organ subgroups: liver, epaxial muscle, pooled viscera (except the liver and brain), and pooled avisceral carcass (muscle, skin, and bone). The total tiger salamander xenobiotic burdens were calculated from these data. The malathion/malaoxon burden 1 day after exposure was greatest in the avisceral carcass and 2 days after exposure was greatest in the viscera. Bioconcentration and bioaccumulation factors remained less than unity throughout the experiment and did not support the hypothesis of bioaccumulation of malathion in the tiger salamander. Biological impact was assessed with a colorimetric brain cholinesterase microassay. Brain cholinesterase activities in salamanders exposed to malathion-contaminated soil (50 μg/cm2 or 100 μg/cm2 malathion) were suppressed ∼50–65% and 90%, respectively, compared to unexposed controls. The exposed animals did not exhibit overt clinical signs of malathion toxicosis.


Similar content being viewed by others
References
Albanis T, Hela D, Papacostas G, et al. (1996) Concentration and bioaccumulation of organochlorine pesticide resides in herons and their prey in wetlands of Thermaikos Gulf, Macedonia, Greece. Sci Total Environ 182:11–19
Alford R, Richards S (2001) Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst 30:133–165
Anguiano O, De Castro AC, D’Angelo A, et al. (2001) The role of glutathion conjugation in the regulation of early toad embryo’s tolerance to pesticides. Comp Biochem Physiol C: Toxicol Pharmacol 128(1):35–43
Baker K (1985) Laboratory and field experiments on the responses by two species of woodland salamanders to malathion-treated substrates. Arch Environ Contam Toxicol 14:685–691
Bentley P, G. Baldwin G (1980) Comparison of transcutaneous permeability in skins of larval and adult salamanders (Ambystoma tigrinum). Am J Physiol 239:R505–R508
Blaustein A, Romansic J, Kiescecker J, et al. (2003) Ultraviolet rationation, toxic chemicals, and amphibian population declines. Divers Distrib 9:123
Bouchard M, Gosselin N, Brunet R, et al. (2003) A toxicokinetic model of malathion and its metabolites as a tool to assess human exposure and risk through measurements or urinary biomarkers. Toxicol Sci 73:182–194
Boutisiouki P, Thompson JP, Clough GF, et al. (2001) Effects of local blood flow on the percutaneous absoption of the organophophorous compound malathion: a microdialysis study in man. Arch Toxicol 75:321–328
Carlock L, Chen WL, Gordon E, et al. (1999) Regulating and assessing risks of cholinesterase-inhibiting pesticides: divergent approaches adn interpretations. J Toxicol EnvironHealth B 2:105–160
Dary C, Blancato C, Castles J, et al. (1993) Dermal absorption and disposition of formulation of malathion in Sprague-Dawley rats and humans. ACS Symp Ser 542: 231
DeGuise S, Maratea J, Perkins C (2004) Malathion immunotoxicity in the American Lobster (Homarus americanus) upon experimental exposure. Aquatic Toxicol 66(4):419–425
Edery H, Schatzberg-Porath G (1960) Studies on the effect of organophosphorus insecticides on amphibians. Arch Int Ther 124:212–224
Ellman G, Courtney KD, Andres VJ, et al. (1961) A new and rapid colorimeteric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
EPA (Environmental Protection Agency) (2000) Malathion: environmental fate and effects. Available from http://www.epa.gov/pesticides/op/malathion.htm (accessed 15 May 2002)
Eto M (1974) Organophosphorus pesticides: Organic and biological chemistry. CRC Press, Cleveland, OH
Folch J, Lees M, Stanley GHS, et al. (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fulton M, Key P (2000) Annual review: Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environl Tox Chem 20(1):37–46
Geyer H, Scheurnert J, Korte F, et al. (1986) Bioconcentration potential of organic environmental chemicals in humans. Regul Toxicol Pharmacol 6:313–347
Guthrie FE, Perry JJ (eds) (1980) Introduction to environmental toxicology. Elsevier, Amsterdam
Hall R, Kolbe E (1980) Bioconcentration of organophosphorus pesticides to hazardous levels by amphibians. J Toxicol Environ Health 6:853–860
Johnson M, Franke L, Lee R, et al. (1999). “Bioaccumulation of 2,4,6-trinitrotoluene and polychlorinated biphenyls through two routes of exposure in a terrestrial amphibian: is the dermal route significant? Environ Toxicol Chem 18(5):873–876
Khan S, Khan N (1986) The mobility of some organophosphorus pesticides in soil as affected by some soil parameters. Soil Sci 142:214–221
Krieger RI, Dinoff TM (2000) Malathion deposition, metabolite clearance, and cholinesterase status of date dusters and harvesters in California. Arch Environ Contam Toxicol 38(4):546–553
Rosenbaum E, de Castro AC, Gauna L, et al. (1988) Early biochemical changes produced by malathion on toad embryos. Arch Environ Contam Toxicol 17:831–835
Sparling D, Fellers G, McConnell L, et al. (2001) Pesticides and amphibian population declines in California, USA. Environ Tox Chem 20(7):1591–1595
Taylor SK, Williams E, Mills K, et al. (1999) Effects of malathion on disease susceptibility in Woodhouse’s toad. J Wildl Dis 35(3):536–541
Tsuda T, Aoki S, Kojima M, et al. (1989) Bioconcentration and excretion of Diazinon, IBP, Malathion, and Fenitrothion by Willow Shiner. Toxicol Environ Chem 24:185–190
Venturino A, Anguino O, Gauna L, et al. (2001) Thiols and polyamines in the potentiation of malathion toxicity in larval stages of the toad Bufo arenarum. Comp Biochem Physiol C 130:191–198
Venturino A, Gauna L, Bergoc R, et al. (2001) Toxicokinetics of malathion in larval stages of the toad Bufo arenarum (Hensel): effect of exogenous spermidine. Pesticide Biochem Physiol 70(3):142–150
Venturino A, Rosenbaum E, Caballero A, et al. (2003) Biomarkers of effect in toads and frogs. Biomarkers 8:167–186
Willens S (2005) Effects of percutaneous malathion absorption in anurans. Comparative Biomedical Sciences. Thesis, North Carolina State University, Raleigh, 108 pp
Acknowledgments
We thank the Environmental Protection Agency for funding this research and express our gratitude for the graphic support of Linda Dunn, NCSU CMAST. The US Environmental Protection Agency (EPA) through its Office of Research and Development partially funded and collaborated in the research described here under assistance agreement #R-83055101 to North Carolina State University. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the EPA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henson-Ramsey, H., Kennedy-Stoskopf, S., Levine, J.F. et al. Acute Toxicity and Tissue Distributions of Malathion in Ambystoma tigrinum . Arch Environ Contam Toxicol 55, 481–487 (2008). https://doi.org/10.1007/s00244-007-9091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-007-9091-4