Abstract
Introduction
It is known that taste is centrally represented in the insula, frontal and parietal operculum, as well as in the orbitofrontal cortex (secondary gustatory cortex). In functional MRI (fMRI) experiments activation in the insula has been confirmed, but activation in the orbitofrontal cortex is only infrequently found, especially at higher field strengths (3 T). Due to large susceptibility artefacts, the orbitofrontal cortex is a difficult region to examine with fMRI. Our aim was to localize taste in the human cortex at 3 T, specifically in the orbitofrontal cortex as well as in the primary gustatory cortex.
Methods
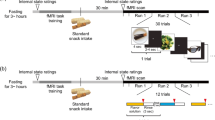
Event-related fMRI was performed at 3 T in seven healthy volunteers. Taste stimuli consisted of lemon juice and chocolate. To visualize activation in the orbitofrontal cortex a dedicated 3D SENSE EPI fMRI sequence was used, in addition to a 2D SENSE EPI fMRI sequence for imaging the entire brain. Data were analyzed using a perception-based model.
Results
The dedicated 3D SENSE EPI sequence successfully reduced susceptibility artefacts in the orbitofrontal area. Significant taste-related activation was found in the orbitofrontal and insular cortices.
Conclusion
fMRI of the orbitofrontal cortex is feasible at 3 T, using a dedicated sequence. Our results corroborate findings from previous studies.






Similar content being viewed by others
References
Kringelbach ML, de Araujo IE, Rolls ET (2004) Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage 21:781–788
Lee BC, Hwang SH, Rison R, Chang GY (1998) Central pathway of taste: clinical and MRI study. Eur Neurol 39:200–203
Penfield W, Faulk ME Jr (1955) The insula; further observations on its function. Brain 78:445–470
Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M (1999) Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport 10:7–14
Faurion A, Cerf B, Le Bihan D, Pillias AM (1998) fMRI study of taste cortical areas in humans. Ann N Y Acad Sci 855:535–545
Frank GK, Kaye WH, Carter CS, Brooks S, May C, Fissell K, Stenger VA (2003) The evaluation of brain activity in response to taste stimuli – a pilot study and method for central taste activation as assessed by event-related fMRI. J Neurosci Methods 131:99–105
Cerf-Ducastel B, Van de Moortele PF, MacLeod P, Le Bihan D, Faurion A (2001) Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses 26:371–383
Norgren R (1990) Gustatory system. In: Paxinos G (ed) The human nervous system. Academic Press, New York, pp 845–861
Zald DH, Hagen MC, Pardo JV (2002) Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol 87:1068–1075
Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D (2004) Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol 92:1892–1903
de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N (2003) Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci 18:2059–2068
O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ (2002) Neural responses during anticipation of a primary taste reward. Neuron 33:815–826
Rolls ET (1999) The brain and emotion. Oxford University Press, Oxford
Ogawa H (1994) Gustatory cortex of primates: anatomy and physiology. Neurosci Res 20:1–13
Rolls ET, Baylis LL (1994) Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci 14:5437–5452
Weiger M, Pruessmann KP, Osterbauer R, Bornert P, Boesiger P, Jezzard P (2002) Sensitivity-encoded single-shot spiral imaging for reduced susceptibility artifacts in BOLD fMRI. Magn Reson Med 48:860–866
Peeters RR, Sunaert S, Smits M, Van Hecke P (2004) Optimization of 3D EPI SENSE techniques for fMRI of highly inhomogeneous areas. In: Proceedings of the International Society for Magnetic Resonance in Medicine 12th Scientific Meeting, Kyoto, p 1028
Calder AJ, Lawrence AD, Young AW (2001) Neuropsychology of fear and loathing. Nat Rev Neurosci 2:352–363
Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H (2000) A neural basis for general intelligence. Science 289:457–460
De Panfilis C, Schwarzbauer C (2005) Positive or negative blips? The effect of phase encoding scheme on susceptibility-induced signal losses in EPI. Neuroimage 25:112–121
Deichmann R, Gottfried JA, Hutton C, Turner R (2003) Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19:430–441
Kruger G, Kastrup A, Glover GH (2001) Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magn Reson Med 45:595–604
Gu H, Feng H, Zhan W, Xu S, Silbersweig DA, Stern E, Yang Y (2002) Single-shot interleaved z-shim EPI with optimized compensation for signal losses due to susceptibility-induced field inhomogeneity at 3 T. Neuroimage 17:1358–1364
Li Z, Wu G, Zhao X, Luo F, Li SJ (2002) Multiecho segmented EPI with z-shimmed background gradient compensation (MESBAC) pulse sequence for fMRI. Magn Reson Med 48:312–321
Deichmann R, Josephs O, Hutton C, Corfield DR, Turner R (2002) Compensation of susceptibility-induced BOLD sensitivity losses in echo-planar fMRI imaging. Neuroimage 15:120–135
Posse S, Shen Z, Kiselev V, Kemna LJ (2003) Single-shot T(2)* mapping with 3D compensation of local susceptibility gradients in multiple regions. Neuroimage 18:390–400
Glover GH, Law CS (2001) Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med 46:515–522
Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH (2004) Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage 21:291–301
Cordes D, Turski PA, Sorenson JA (2000) Compensation of susceptibility-induced signal loss in echo-planar imaging for functional applications. Magn Reson Imaging 18:1055–1068
Hsu JJ, Glover GH (2005) Mitigation of susceptibility-induced signal loss in neuroimaging using localized shim coils. Magn Reson Med 53:243–248
Cusack R, Russell B, Cox SM, De Panfilis C, Schwarzbauer C, Ansorge R (2005) An evaluation of the use of passive shimming to improve frontal sensitivity in fMRI. Neuroimage 24:82–91
Wilson JL, Jezzard P (2003) Utilization of an intra-oral diamagnetic passive shim in functional MRI of the inferior frontal cortex. Magn Reson Med 50:1089–1094
Osterbauer RA, Wilson JL, Calvert GA, Jezzard P (2006) Physical and physiological consequences of passive intra-oral shimming. Neuroimage 29:245–253
Wilson JL, Jenkinson M, de Araujo I, Kringelbach ML, Rolls ET, Jezzard P (2002) Fast, fully automated global and local magnetic field optimization for fMRI of the human brain. Neuroimage 17:967–976
Preibisch C, Pilatus U, Bunke J, Hoogenraad F, Zanella F, Lanfermann H (2003) Functional MRI using sensitivity-encoded echo planar imaging (SENSE-EPI). Neuroimage 19:412–421
Wang Y (2000) Description of parallel imaging in MRI using multiple coils. Magn Reson Med 44:495–499
De Zwart JA, Ledden PJ, Van Gelderen P, Bodurka J, Chu R, Duyn JH (2004) Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med 51:22–26
Schmidt CF, Degonda N, Luechinger R, Henke K, Boesiger P (2005) Sensitivity-encoded (SENSE) echo planar fMRI at 3T in the medial temporal lobe. Neuroimage 25:625–641
Weiger M, Pruessmann KP, Boesiger P (2002) 2D SENSE for faster 3D MRI. Magma 14:10–19
McCarthy G, Allison T, Spencer DD (1993) Localization of the face area of human sensorimotor cortex by intracranial recording of somatosensory evoked potentials. J Neurosurg 79:874–884
Iannetti GD, Porro CA, Pantano P, Romanelli PL, Galeotti F, Cruccu G (2003) Representation of different trigeminal divisions within the primary and secondary human somatosensory cortex. Neuroimage 19:906–912
Cerf-Ducastel B, Murphy C (2004) Improvement of fMRI data processing of olfactory responses with a perception-based template. Neuroimage 22:603–610
Faurion A, Cerf B, Van De Moortele PF, Lobel E, MacLeod P, Le Bihan D (1999) Human taste cortical areas studied with functional magnetic resonance imaging: evidence of functional lateralization related to handedness. Neurosci Lett 277:189–192
Cerf B, Lebihan D, Van de Moortele PF, MacLeod P, Faurion A (1998) Functional lateralization of human gustatory cortex related to handedness disclosed by fMRI study. Ann N Y Acad Sci 855:575–578
Acknowledgements
This study was made possible with funding from a European Union Marie-Curie Fellowship and from “Fonds voor Wetenschappelijk Onderzoek (FWO)-Flanders Wetenschappelijke Onderzoeksgemeenschap (WOG) on Advanced Nuclear Magnetic Resonance (NMR)”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smits, M., Peeters, R.R., van Hecke, P. et al. A 3 T event-related functional magnetic resonance imaging (fMRI) study of primary and secondary gustatory cortex localization using natural tastants. Neuroradiology 49, 61–71 (2007). https://doi.org/10.1007/s00234-006-0160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0160-6