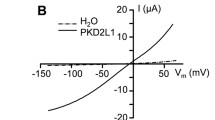
Abstract
Certain antimicrobial peptides from multicellular animals kill a variety of tumor cells at concentrations not affecting normal eukaryotic cells. Recently, it was reported that also plantaricin A (PlnA), which is a peptide pheromone with strain-specific antibacterial activity produced by Lactobacillus plantarum, permeabilizes cancerous rat pituitary cells (GH4 cells), whereas normal rat anterior pituitary cells are resistant to the peptide. To examine whether the preferential permeabilization of cancerous cells is a general feature of PlnA, we studied its effect on primary cultures of cells from rat liver (hepatocytes, endothelial, and Kupffer cells) and rat kidney cortex, as well as two epithelial cell lines of primate kidney origin (Vero cells from green monkey and human Caki-2 cells). The Vero cell line is derived from normal cells, whereas the Caki-2 cell line is derived from a cancerous tumor. The membrane effects were studied by patch clamp recordings and microfluorometric (fura-2) monitoring of the cytosolic concentrations of Ca2+ ([Ca2+]i) and fluorophore. In all the tested cell types except Kupffer cells, exposure to 10–100 μM PlnA induced a nearly instant permeabilization of the membrane, indicated by the following criteria: increased membrane conductance, membrane depolarization, increased [Ca2+]i, and diffusional loss of fluorophore from the cytosol. At a concentration of 5 μM, PlnA had no effect on any of the cell types. The Kupffer cells were permeabilized by 500 μM PlnA. We conclude that the permeabilizing effect of PlnA is not restricted to cancerous cells.




Similar content being viewed by others
References
Anderssen EL, Diep DB, Nes IF, Eijsink VG, Nissen-Meyer J (1998) Antagonistic activity of Lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl Environ Microbiol 64:2269–2272
Balasubramanian K, Schroit AJ (2003) Aminophospholipid asymmetry: a matter of life and death. Annu Rev Physiol 65:701–734
Blomhoff R, Smedsrød B, Eskild W, Granum PE, Berg T (1984) Preparation of isolated liver endothelial cells and Kupffer cells in high yield by means of an enterotoxin. Exp Cell Res 150:194–204
Castano S, Desbat B, Delfour A, Dumas JM, da Silva A, Dufourcq J (2005) Study of structure and orientation of mesentericin Y105, a bacteriocin from gram-positive Leuconostoc mesenteroides, and its Trp-substituted analogues in phospholipid environments. Biochim Biophys Acta 1668:87–98
Chen HM, Wang W, Smith D, Chan SC (1997) Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochim Biophys Acta 1336:171–179
Chen HM, Leung KW, Thakur NN, Tan A, Jack RW (2003) Distinguishing between different pathways of bilayer disruption by the related antimicrobial peptides cecropin B, B1 and B3. Eur J Biochem 270:911–920
Cruciani RA, Barker JL, Zasloff M, Chen HC, Colamonici O (1991) Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci USA 88:3792–3796
Diep DB, Havarstein LS, Nissen-Meyer J, Nes IF (1994) The gene encoding plantaricin A, a bacteriocin from Lactobacillus plantarum C11, is located on the same transcription unit as an agr-like regulatory system. Appl Environ Microbiol 60:160–166
Diep DB, Havarstein LS, Nes IF (1995) A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol Microbiol 18:631–639
Fimland G, Johnsen L, Dalhus B, Nissen-Meyer J (2005) Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action. J Pept Sci 11:688–696
Fogh J, Fogh JM, Orfeo T (1977) One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 58:209–214
Hafting T, Haug TM, Ellefsen S, Sand O (2006) Hypotonic stress activates BK channels in clonal kidney cells via purinergic receptors, presumably of the P2Y1 subtype. Acta Physiol 188:21–31
Hamill O, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hancock RE, Chapple DS (1999) Peptide antibiotics. Antimicrob Agents Chemother 43:1317–1323
Hauge HH, Mantzilas D, Moll GN, Konings WN, Driessen AJ, Eijsink VG, Nissen-Meyer J (1998) Plantaricin A is an amphiphilic alpha-helical bacteriocin-like pheromone which exerts antimicrobial and pheromone activities through different mechanisms. Biochemistry 37:16026–16032
Jacob L, Zasloff M (1994) Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. Ciba Found Symp 186:197–216
Kristiansen PE, Fimland G, Mantzilas D, Nissen-Meyer J (2005) Structure and mode of action of the membrane-permeabilizing antimicrobial peptide pheromone plantaricin A. J Biol Chem 280:22945–22950
Lehrer RI, Lichtenstein AK, Ganz T (1993) Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 11:105–128
Leuschner C, Hansel W (2004) Membrane disrupting lytic peptides for cancer treatments. Curr Pharm Des 10:2299–2310
Lichtenstein A (1991) Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J Clin Invest 88:93–100
Liebhaber H, Riordan JT, Horstmann DM (1967) Replication of rubella virus in a continuous line of African green monkey kidney cells (Vero). Proc Soc Exp Biol Med 125:636–643
Matsuzaki K (1998) Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim Biophys Acta 1376:391–400
Matsuzaki K (1999) Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta 1462:1–10
Matsuzaki K, Sugishita K, Fujii N, Miyajima K (1995) Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry 34:3423–3429
Nissen-Meyer J, Nes IF (1997) Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol 167:67–77
Oren Z, Shai Y (1998) Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 47:451–463
Rao LV, Tait JF, Hoang AD (1992) Binding of annexin V to a human ovarian carcinoma cell line (OC-2008). Contrasting effects on cell surface factor VIIa/tissue factor activity and prothrombinase activity. Thromb Res 67:517–531
Sablon E, Contreras B, Vandamme E (2000) Antimicrobial peptides of lactic acid bacteria: mode of action, genetics and biosynthesis. Adv Biochem Eng Biotechnol 68:21–60
Sand SL, Haug TM, Nissen-Meyer J, Sand O (2007) The bacterial peptide pheromone plantaricin A permeabilizes cancerous, but not normal, rat pituitary cells and differentiates between the outer and inner membrane leaflet. J Membr Biol 216:61–71
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane–lytic peptides. Biochim Biophys Acta 1462:55–70
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236–248
Sugimura M, Donato R, Kakkar VV, Scully MF (1994) Annexin V as a probe of the contribution of anionic phospholipids to the procoagulant activity of tumour cell surfaces. Blood Coagul Fibrinolysis 5:365–373
Tossi A, Sandri L, Giangaspero A (2000) Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4–30
Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ (1991) Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res 51:3062–3066
Williamson P, Schlegel RA (1994) Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol 11:199–216
Ye JS, Zheng XJ, Leung KW, Chen HM, Sheu FS (2004) Induction of transient ion channel-like pores in a cancer cell by antibiotic peptide. J Biochem 136:255–259
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA 84:5449–5453
Zelezetsky I, Pacor S, Pag U, Papo N, Shai Y, Sahl HG, Tossi A (2005) Controlled alteration of the shape and conformational stability of alpha-helical cell–lytic peptides: effect on mode of action and cell specificity. Biochem J 390:177–188
Zhao H, Sood R, Jutila A, Bose S, Fimland G, Nissen-Meyer J, Kinnunen PK (2006) Interaction of the antimicrobial peptide pheromone plantaricin A with model membranes: implications for a novel mechanism of action. Biochim Biophys Acta 1758:1461–1474
Acknowledgments
This work was supported by grants from the Norwegian Research Council. We thank Jon Nissen-Meyer for providing the PlnA and Seyed Ali Mousavi for help in preparing the primary cultures of liver cells.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kristin Andersland and Guro F. Jølle contributed equally to this study.
Rights and permissions
About this article
Cite this article
Andersland, K., Jølle, G.F., Sand, O. et al. Peptide Pheromone Plantaricin A Produced by Lactobacillus plantarum Permeabilizes Liver and Kidney Cells. J Membrane Biol 235, 121–129 (2010). https://doi.org/10.1007/s00232-010-9263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9263-4