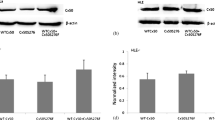
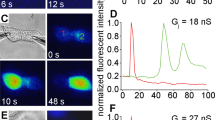
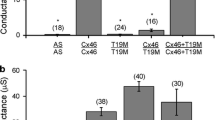
Abstract
Mefloquine (MFQ) selectively blocks exogenously expressed gap junction channels composed of C×50 but not C×46. The purpose of the current study was to evaluate MFQ effects on wild-type (WT) mouse lenses that express both C×50 and C×46 in their outer shell of differentiating fibers (DFs). Lenses in which C×46 was knocked into both C×50 alleles (KI) were used as controls; MFQ had no effect on coupling in these lenses. When WT lenses were exposed to MFQ, the DF coupling conductance decreased significantly, suggesting that C×50 contributes about 57% of the coupling conductance in DF and C×46 contributes 43%. Remarkably, in the presence of MFQ, the 43% of the channels that remained open did not gate closed in response to a reduction in pH, whereas in the absence of MFQ, the same pH change caused all the DF channels to gate closed. Since MFQ is a selective blocker of C×50 channels, it appears that C×46 channels lack pH-mediated gating in the absence of functional C×50 channels but are pH-sensitive in the presence of C×50 channels. These results suggest the two types of channels interact and gate cooperatively.







Similar content being viewed by others
References
Baldo G.J., Gong X., Martinez-Wittinghan F.J., Kumar N.M., Gilula N.B., Mathias R.T. 2001. Gap junctional coupling in lenses from alpha(8) connexin knockout mice. J. Gen. Physiol. 118:447–456
Baldo G.J., Mathias R.T. 1992. Spatial variations in membrane properties in the intact rat lens. Biophys. J. 63:518–529
Cruikshank S.J., Hopperstad M., Younger M., Connors B.W., Spray D.C., Srinivas M. 2004. Potent block of C×36 and C×50 gap junction channels by mefloquine. Proc. Natl. Acad. Sci. USA 101:12364–12369
Eckert R. 2002. pH gating of lens fibre connexins. Pfluegers Arch. 443:843–851
Ek J.F., Delmar M., Perzova R., Taffet S.M. 1994. Role of histidine 95 on pH gating of the cardiac gap junction protein connexin43. Circ. Res. 74:1058–1064
Ek-Vitorin J.F., Calero G., Morley G.E., Coombs W., Taffet S.M., Delmar M. 1996. pH regulation of connexin43: Molecular analysis of the gating particle. Biophys. J. 71:1273–1284
Gonen T., Cheng Y., Sliz P., Hiroaki Y., Fujiyoshi Y., Harrison S.C., Walz T. 2005. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438:633–638
Gonen T., Sliz P., Kistler J., Cheng Y., Walz T. 2004. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 429:193–197
Gong X., Baldo G.J., Kumar N.M., Gilula N.B., Mathias R.T. 1998. Gap junctional coupling in lenses lacking alpha3 connexin. Proc. Natl. Acad. Sci. USA 95:15303–15308
Hopperstad M.G., Srinivas M., Spray D.C. 2000. Properties of gap junction channels formed by C×46 alone and in combination with C×50. Biophys. J. 79:1954–1966
Jiang J.X., Goodenough D.A. 1996. Heteromeric connexons in lens gap junction channels. Proc. Natl. Acad. Sci. USA 93:1287–1291
Kistler J., Christie D., Bullivant S. 1988. Homologies between gap junction proteins in lens, heart and liver. Nature 331:721–723
Konig N., Zampighi G.A. 1995. Purification of bovine lens cell-to-cell channels composed of connexin44 and connexin50. J. Cell Sci. 108:3091–3098
Lin J.S., Fitzgerald S., Dong Y., Knight C., Donaldson P., Kistler J. 1997. Processing of the gap junction protein connexin50 in the ocular lens is accomplished by calpain. Eur. J. Cell Biol. 73:141–149
Manivannan K., Mathias R.T., Gudowska-Nowak E. 1996. Description of interacting channel gating using a stochastic Markovian model. Bull. Math Biol. 58:141–174
Mathias R., Rae J.L., Baldo G.J. 1997. Physiological properties of the normal lens. Physiol. Rev. 77:21–50
Mathias R.T., Rae J.L., Eisenberg R.S. 1981. The lens as a nonuniform spherical syncytium. Biophys. J. 34:61–83
Mathias R.T., Riquelme G., Rae J.L. 1991. Cell to cell communication and pH in the frog lens. J. Gen. Physiol. 98:1085–1103
Martinez-Wittinhan F.J., Sellitto C., White T.W., Mathias R.T., Paul D., Goodenough D.A. 2004. Lens gap junctional coupling is modulated by connexin identity and the locus of gene expression. Invest. Ophthalmol. Vis. Sci. 45:3629–3637
Morley G.E., Taffet S.M., Delmar M. 1996. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys. J. 70:1294–1302
Motten A.G., Martinez L.J., Holt N., Sik R.H., Reszka K., Chignell C.F., Tonnesen H.H., Roberts J.E. 1999. Photophysical studies on antimalarial drugs. Photochem. Photobiol. 69:282–287
Nielsen P.A., Baruch A., Shestopalov V.I., Giepmans B.N., Dunia I., Benedetti E.L., Kumar N.M. 2003. Lens connexins alpha3C×46 and alpha8C×50 interact with zonula occludens protein-1 (ZO-1). Mol. Biol. Cell 14:2470–2481
Peracchia C., Bernardini G. 1984. Gap junction structure and cell-to-cell coupling regulation: Is there a calmodulin involvement? Fed. Proc. 43:2681–2691
Peracchia C., Wang X.C. 1997. Connexin domains relevant to the chemical gating of gap junction channels. Braz. J. Med. Biol. Res. 30:577–590
Spray D.C., Harris A.L., Bennett M.V. 1981. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 211:712–715
Spray D.C., Stern J.H., Harris A.L., Bennett M.V. 1982. Gap junctional conductance: Comparison of sensitivities to H and Ca ions. Proc. Natl. Acad. Sci. USA 79:441–445
Srinivas M., Costa M., Gao Y., Fort A., Fishman G.I., Spray D.C. 1999. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J. Physiol. 517:673–689
Stergiopoulos K., Alvarado J.L., Mastroianni M., Ek-Vitorin J.F., Taffet S.M., Delmar M. 1999. Hetero-domain interactions as a mechanism for the regulation of connexin channels. Circ. Res. 84:1144–1155
Trexler E.B., Bukauskas F.F., Bennett M.V., Bargiello T.A., Verselis V.K. 1999. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J. Gen. Physiol. 113:721–742
Valiunas V., Weingart R. 2001. Co-operativity between mouse connexin30 gap junction channels. Pfluegers Arch. 441:756–760
White T.W. 2002. Unique and redundant connexin contributions to lens development. Science 295:319–320
White T.W., Bruzzone R., Wolfram S., Paul D.L., Goodenough D.A. 1994. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: The second extracellular domain is a determinant of compatibility between connexins. J. Cell Biol. 125:879–892
Yamaguchi D.T., Huang J.T., Ma D. 1995. Regulation of gap junction intercellular communication by pH in MC3T3-E1 osteoblastic cells. J. Bone Miner. Res. 10:1891–1899
Acknowledgement
This work was supported by National Eye Institute grants EY06391, EY13163 and EY13869.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez-Wittinghan, F.J., Srinivas, M., Sellitto, C. et al. Mefloquine Effects on the Lens Suggest Cooperative Gating of Gap Junction Channels. J Membrane Biol 211, 163–171 (2006). https://doi.org/10.1007/s00232-006-0021-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-006-0021-6