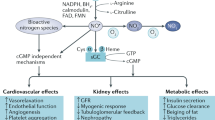
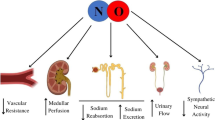
Abstract
There is clear evidence that chronic inhibition of nitric oxide synthase (NOS) in animals causes hypertension and also leads to progressive kidney damage. There is also evidence that nitric oxide (NO) deficiency occurs in man with chronic kidney disease (CRD) and this may contribute to further progression of CRD, to hypertension, and to other cardiovascular complications. There are multiple ways in which NO deficiency develops in CRD. At end stage there are uremic factors in plasma that inhibit L-arginine transport into cells and this may cause a “net” substrate deficiency. Also, increases occur in endogenous NOS inhibitors, in particular asymmetric dimethylarginine (ADMA). The increased oxidative stress of CRD is likely to be a primary cause of the increased plasma ADMA since the catabolic enzyme, dimethylarginine dimethylaminohydrolase (DDAH) is extremely sensitive to inhibition by oxidants. Animal studies demonstrate a decrease in abundance of the neuronal NOS within the injured kidney that correlates with extent of injury. Overall, there is substantial clinical, “in vitro,” and animal data to suggest that systemic, endothelial, and renal NO deficiency is a common feature of CRD irrespective of the primary genesis of the disease. This NO deficiency, which is multifactorial, contributes to the progressive nature of the CRD and the endothelial dysfunction and associated risk for cardiovascular events. Strategies that reverse NOS inhibition and/or can boost the ability of the damaged kidney to produce NO might help preserve residual renal function and/or slow down the rate of progression to end stage.








Similar content being viewed by others
References
Zatz R, Baylis C (1998) Chronic nitric oxide inhibition model six years on. Hypertension 32:958-964
Kone BC, Baylis C (1997) Biosynthesis and homeostatic roles of nitric oxide in the kidney. Editorial review. Am J Physiol 272:F561–F578
Schmidt R, Domico J, Samsell L, Yokota S, Tracy T, Sorkin M, Engels K, Baylis C (1999) Indices of activity of the nitric oxide system in patients on hemodialysis. Am J Kidney Dis 34:228–234
Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C (1999) Nitric oxide production is low in end stage renal disease patients on peritoneal dialysis. Am J Physiol 276:F794–F797
Schmidt RJ, Baylis C (2000) Total nitric oxide production is low in patients with chronic renal disease. Kidney Int 58:1261–1266
Baylis C, Vallance P (1998) Measurement of nitrite and nitrate (NOx) levels in plasma and urine: what does this measure tell us about the activity of the endogenous nitric oxide? Curr Opin Nephrol Hypertens 7:59–62
Blum M, Yachnin T, Wollman Y, Chernihovsky T, Peer G, Grosskopf I, Kaplan E, Silverberg D, Cabili S, Iaina A (1998) Low nitric oxide production in patients with chronic renal failure. Nephron 79:265–268
Wever R, Boer P, Hijmering M, Stroes E, Verhaar M, Kastelein J, Versluis K, Lagerwerf F, van Rijn H, Koomans H, Rabelink T (1999) Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 19:1168–1172
Lau T, Owen W, Yu YM, Noviski N, Lyons J, Zurakowski D, Tsay R, Ajami A, Young VR, Castillo L (2000) Arginine, citrulline, and nitric oxide metabolism in end-stage renal disease patients. J Clin Invest 105:1217–1225
Erdely A, Freshour G, Smith C, Engels K, Olson J, Baylis C (2003) Protection against puromycin aminonucleoside (PAN) induced chronic renal disease (CRD) in the Wistar Furth (WF). J Am Soc Nephrol 14:155A
Erdely A, Greenfeld Z, Wagner L, Baylis C (2003) Sexual dimorphism in the aging kidney; inverse relationship between injury and nitric oxide system. Kidney Int 63:1021–1026
Erdely A, Wagner L, Muller V, Szabo A, Baylis C (2003) Protection of Wistar Furth rat from chronic renal disease is associated with maintained renal nitric oxide synthase vs. Sprague Dawley. J Am Soc Nephrol 14:2526–2533
Wagner L, Klein J, Sands J, Baylis C (2002) Urea transporters are widely distributed in endothelial cells and mediate inhibition of L-arginine transport. Am J Physiol 283:F578–F582
Xiao S, Schmidt RJ, Baylis C (2000) Plasma from ESRD patients inhibits nitric oxide synthase (NOS) activity in cultured human and bovine endothelial cells. Acta Physiol Scand 168:175–179
Arnal JF, Warin L, Michel JB (1992) Determinants of aortic cyclic guanosine monophosphate in hypertension induced by chronic inhibition of nitric oxide synthase. J Clin Invest 90:647–652
Morris SM Jr (2000) Regulation of arginine availability and its impact on NO synthesis. In: Ignarro LJ (ed) Nitric oxide, biology and pathobiology. Academic, San Diego, pp 187–197
Mistry S, Greenfeld Z, Morris S, Baylis C (2002) The “intestinal-renal” arginine biosynthetic axis in the aging rat. Mech Age Dev 123:1159–1165
Xiao S, Wagner L, Baylis C (2001) Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am J Physiol 49:F989–F995
McDonald KK, Zharikov S, Block ER, Kilberg MS (1997) A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem 272:31213–31216
Vallance P, Leon A, Calver A, Collier J, Moncada S (1992) Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339:572–575
Anderstam B, Katzarski K, Bergstrom J (1997) Serum levels of NG, NG-dimethyl-L arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol 8:1437–1442
Pettersson A, Uggla L, Bachman V (1997) Determination of dimethylated arginines in human plasma by high pressure liquid chromatography. J Chromatogr B 692:257–262
Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Böger RH, CREED Investigators (2002) Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62:339–345
Kielstein JT, Böger RH, Bode-Böger SM, Frölich JC, Haller H, Ritz E, Fliser D (2002) Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol 13:170–176
Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P (2003) Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23:1455–1459
Calver A, Collier J, Leone A, Moncada S, Vallance P (1993) Effect of local intra-arterial asymmetric dimethylarginine (ADMA) on the forearm arteriolar bed of healthy volunteers. J Hum Hypertens 7:193–194
Xiao S, Wagner L, Schmidt RJ, Baylis C (2001) Circulating eNOS inhibitory factor in some patients with chronic renal disease. Kidney Int 59:1466–1472
Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS (1997) Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int 52:1593–1601
MacAllister RJ, Parry H, Kimoto M, Ogawa T, Russell RJ, Hodson H, Whitley GST, Vallance P (1996) Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol 119:1533–1540
Jones LC, Tran CT, Leiper JM, Hingorani AD, Vallance P (2003) Common genetic variation in a basal promoter element alters DDAH2 expression in endothelial cells. Biochem Biophys Res Commun 310:836–843
Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP (1998) Asymmetric dimethylarginine: a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98:1842–1847
Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP (1999) Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 99:3092–3095
Himmelfarb J (2004) Linking oxidative stress and inflammation in kidney disease: which is the chicken and which is the egg? Semin Dial 17:449–454
Fickling SA, Holden DP, Cartwright JE, Nussey SS, Vallance P, Whitley GS (1999) Regulation of macrophage nitric oxide synthesis by endothelial cells: a role for NG, NG-dimethylarginine. Acta Physiol Scand 167:145–150
Kone BC, Kuncewicz T, Zhang W, Yu ZY (2003) Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol 285:F178–F190
Roczniak A, Levine DZ, Burns KD (2000) Localization of protein inhibitor of neuronal nitric oxide synthase in rat kidney. Am J Physiol 278:F702–F707
Aiello S, Noris M, Todeschini M, Zappella S, Foglieni C, Benigni A, Corna D, Zoja C, Cavallotti D, Remuzzi G (1997) Renal and systemic nitric oxide synthesis in rats with renal mass reduction. Kidney Int 52:171–181
Vaziri ND, Ni Z, Wang XQ, Oveisi F, Zhou XL (1998) Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH. Am J Physiol 274:F642–F649
Szabo A, Wagner L, Erdely A, Lau K, Baylis C (2003) Renal neuronal nitric oxide synthase protein expression as a marker of renal function. Kidney Int 64:1765–1771
Wagner L, Riggleman A, Erdely A, Couser W, Baylis C (2002) Reduced NOS activity in rats with chronic renal disease due to glomerulonephritis. Kidney Int 62:532–536
Müller V, Szabó A, Baylis C (2002) Changes in renal endothelial and neuronal nitric oxide synthase (eNOS, nNOS) protein abundance in chronic allograft rejection in rats. J Am Soc Nephrol 13:558A
Fitzgibbon WR, Greene EL, Grewal JS, Hutchison FN, Self SE, Latten SY, Ullian ME (1999) Resistance to remnant nephropathy in the Wistar-Furth rat. J Am Soc Nephrol 10:814–821
Müller V, Engels K, Baylis C (2003) Chronic inhibition of nitric oxide synthase (NOS) renders the C57BL6 mouse susceptible to the development of chronic renal disease. J Am Soc Nephrol 14:625A
Acknowledgements
I thank past and present members of my laboratory who have contributed to various aspects of this work. This work has been funded by NIH grants HL 31933, DK 45517 and DK 56843
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baylis, C. Nitric oxide deficiency in chronic renal disease. Eur J Clin Pharmacol 62 (Suppl 1), 123–130 (2006). https://doi.org/10.1007/s00228-005-0003-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0003-0