Abstract
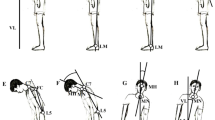
Involuntary movements such as levodopa-induced dyskinesia in Parkinson’s disease (PD) and chorea in Huntington’s disease (HD) are the consequence of two distinct basal ganglia dysfunctions. Yet, their clinical manifestations seem to resemble each other. We seek to determine how to detect PD dyskinesia and HD chorea during quiet stance using healthy control subjects’ postural sway as a base measure and identify means to distinguish mathematically HD chorea from PD dyskinesia. Movements were recorded using a magnetic tracker system with fifteen sensors placed strategically to capture whole-body displacement. Choreic and dyskinetic patients as well as healthy controls were asked to stand with arms stretched horizontally in front of them for 60 s. We examined amplitude, frequency dispersion, proportional energy, sample entropy, kurtosis, skewness, amplitude fluctuation, maximum coherency between 44 pairs of body segments. The choreic and dyskinetic movements revealed similar patterns of sample entropy, amplitude fluctuation, and coherencies between body segments. However, skewness and kurtosis for velocity of movements were found to be higher in HD chorea than in PD dyskinesia, reflecting rapid movements in HD patients. There was also a tendency for the frequency composition of PD dyskinesia to be more concentrated in the 1.0–1.5 Hz range. Our results show that despite their similarities in apparent randomness and lack of coordination, dyskinesia associated with treatment of PD and chorea in HD each have their own distinctive characteristics which may be related to their specific pathophysiology.











Similar content being viewed by others
References
Aubert I, Guigoni C, Hakansson K et al (2005) Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57:17–26
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Statist 29(4):1165–1188
Beuter A, Edwards R (1999) Using frequency domain characteristics to discriminate physiologic and parkinsonian tremors. J Clin Neurophysiol 16(5):484–494
Beuter A, Edwards R, Titcombe MS (2003) Data analysis and mathematical modeling of human tremor. In: Beuter A, Glass L, Mackey MC, Titcombe MS (eds) Nonlinear dynamics in physiology and medicine. Springer, Berlin, pp 303–350
Carignan B, Daneault JF, Duval C (2011) Assessing levodopa-induced dyskinesia in the clinic, the laboratory and the natural environment of patients. J Park Dis 1(4):329–337
Carta AR, Tronci E, Pinna A, Morelli M (2005) Different responsiveness of striatonigral and striatopallidal neurons to L-DOPA after a subchronic intermittent L-DOPA treatment. Eur J Neurosci 21:1196–1204
Cenci A (2007) Dopamine dysregulation of movement control in l-dopa-induced dyskinesia. Trends Neurosci 30:236–243
Devore JL (2004) Probability and statistics for engineering and the sciences. Duxbury, Boston
Duval C, Jones J (2005) Assessment of the amplitude of oscillations associated with high-frequency components of physiological tremor: impact of loading and signal differentiation. Exp Brain Res 163:261–266
Duval C, Fenney A, Jog MS (2009) The dynamic relationship between voluntary and involuntary motor behaviours in patients with movement disorders. In: Groenewegen HJ (ed) Advances in behavioral biology. The Basal Ganglia IX 58:521–534
Fenney A, Jog MS, Duval C (2008a) Bradykinesia is not a “systematic” feature of adult-onset Huntington’s disease; implications for basal ganglia pathophysiology. Brain Res 1193:67–75
Fenney A, Jog MS, Duval C (2008b) Short-term variability in amplitude and motor topography of whole-body involuntary movements in Parkinson’s disease dyskinesias and in Huntington’s chorea. Clin Neurol Neurosurg 110:160–167
Ghassemi M, Lemieux S, Jog M, Edwards R, Duval C (2006) Bradykinesia in patients with Parkinson’s disease having levodopa-induced dyskinesias. Brain Res Bull 69(5):512–518
Gour J, Edwards R, Lemieux S, Ghassemi M, Jog M, Duval C (2007) Movement patterns of peak-dose levodopa-induced dyskinesias in patients with Parkinson’s disease. Brain Res Bull 74:66–74
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Statist 6(2):65–70
Keijsers NL, Horstink MW, Gielen SC (2003) Automatic assessment of levodopa-induced dyskinesias in daily life by neural networks. Mov Disord 18(1):70–80
Kennel M, Brown R, Abarbanel H (1992) Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys Rev 45:3403–3411
Lake DE, Richman JS, Griffin MP, Moorman JP (2002) Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol 52(3):R789–R797
Lemieux S, Ghassemi M, Jog M, Edwards R, Duval C (2007) The influence of levodopa-induced dyskinesias on manual tracking in patients with Parkinson’s disease. Exp Brain Res 176(3):465–475
Mann RK, Edwards R, Zhou J, Jog M, Duval C (2010) Intra- and inter-limb coherency during stance in non-dyskinetic and dyskinetic patients with Parkinson’s disease. Clin Neurol and Neurosurg 112(5):392–399
Norman K, Edwards R, Beuter (1999) The measurement of tremor using a velocity transducer: comparison to simultaneous recordings using transducers of displacement, acceleration and muscle activity. J Neuros Methods 92:41–54
Reiner A, Albin RL, Anderson KD (1988) Differential loss of striatal projection neurons in Huntington’s disease. Proc Natl Acad Sci USA 85:5733–5737
Richman J, Moorman J (2000) Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol 278(6):H2039–H2049
Shumway R, Stoffer D (2006) Time series analysis and its applications with R examples, 2nd edn. Springer, Berlin
Strassburger K, Bretz F (2008) Compatible simultaneous lower confidence bounds for the Holm procedure and other Bonferroni-based closed tests. Stat Med 27(4):4914–4927
van den Hoff JI, Plas AA, Wagemans EA, van Hilten JJ (2001) Accelerometric assessment of levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord 16(1):58–61
Acknowledgments
The authors would like to thank the subjects who graciously participated in the study. This study was funded by Parkinson Society Canada, the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation and the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mann, R.K., Edwards, R., Zhou, J. et al. Comparing movement patterns associated with Huntington’s chorea and Parkinson’s dyskinesia. Exp Brain Res 218, 639–654 (2012). https://doi.org/10.1007/s00221-012-3057-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3057-0