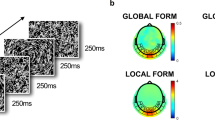
Abstract
There are important developmental changes occurring during infancy in visual cortical structures that underlie higher-order perceptual abilities. Using high-density electrophysiological recording techniques, the present study aimed to examine the development of visual mechanisms, during the first year of life, associated with texture segregation. Forty-two normal full term infants were tested at 1, 3, 6 or 12 months of age. Visual-evoked potentials to low-level stimuli varying in orientation (oriVEP) and higher-level textured stimuli (texVEP) were recorded from 128 scalp electrodes. Difference potentials were obtained to extract the VEP component associated specifically with texture segregation (tsVEP). Results show a clear developmental pattern regarding amplitude, latency and scalp distribution of tsVEP, which appears at around 3 months but does not reach maturity by 12 months of age. A reduction in latency is particularly evident between 3 and 6 months, whereas amplitude shows a gradual increase with a marked increment between 3 and 6 months for low-level orientation stimuli and between 6 and 12 months for higher-level textured stimuli. These developmental patterns are attributed to neural maturational processes such as myelination and synaptogenesis. The differential developmental rates can be explained by delayed maturational processes of brain regions involved in more complex visual processing.






Similar content being viewed by others
References
Atkinson J, Braddick O (1992) Visual segmentation of oriented textures by infants. Behav Brain Res 49(1):123–131
Bach M, Meigen T (1990) Electrophysiological correlates of texture-segmentation in human observers. Invest Ophthalmol Vis Sci 31:104
Bach M, Meigen T (1992) Electrophysiological correlates of texture segregation in the human visual evoked potential. Vision Res 32(3):417–424
Bach M, Meigen T (1997) Similar electrophysiological correlates of texture segregation induced by luminance, orientation, motion and stereo. Vision Res 37(11):1409–1414
Bach M, Schmitt C, Quenzer T, Meigen T, Fahle M (2000) Summation of texture segregation across orientation and spatial frequency: electrophysiological and psychophysical findings. Vision Res 40(26):3559–3566
Barnet AB, Friedman SL, Weiss IP, Ohlrich ES, Shanks B, Lodge A (1980) VEP development in infancy and early childhood. A longitudinal study. Electroencephalogr Clin Neurophysiol 49(5–6):476–489
Bourne JA, Rosa MG (2006) Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT). Cereb Cortex 16(3):405–414
Braddick OJ, Wattam-Bell J, Atkinson J (1986) Orientation-specific cortical responses develop in early infancy. Nature 320(6063):617–619
Burkhalter A (1993) Development of forward and feedback connections between areas V1 and V2 of human visual cortex. Cereb Cortex 3(5):476–487
Cheour M, Leppanen PH, Kraus N (2000) Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clin Neurophysiol 111(1):4–16
Crognale MA (2002) Development, maturation, and aging of chromatic visual pathways: VEP results. J Vis 2(6):438–450
Distler C, Bachevalier J, Kennedy C, Mishkin M, Ungerleider LG (1996) Functional development of the corticocortical pathway for motion analysis in the macaque monkey: a 14C-2-deoxyglucose study. Cereb Cortex 6(2):184–195
Dobson V, Teller DY (1978) Visual acuity in human infants: a review and comparison of behavioral and electrophysiological studies. Vision Res 18(11):1469–1483
Doucet ME, Gosselin F, Lassonde M, Guillemot JP, Lepore F (2005) Development of visual-evoked potentials to radially modulated concentric patterns. Neuroreport 16(16):1753–1756
Eggermont JJ (1988) On the rate of maturation of sensory evoked potentials. Electroencephalogr Clin Neurophysiol 70(4):293–305
Fahle M, Quenzer T, Braun C, Spang K (2003) Feature-specific electrophysiological correlates of texture segregation. Vision Res 43(1):7–19
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101(21):8174–8179
Gratton G, Coles MG, Donchin E (1983) A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55(4):468–484
Hammarrenger B, Lepore F, Lippe S, Labrosse M, Guillemot JP, Roy MS (2003) Magnocellular and parvocellular developmental course in infants during the first year of life. Doc Ophthalmol 107(3):225–233
Huttenlocher PR (1984) Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic 88(5):488–496
Huttenlocher PR, de Court (1987) The development of synapses in striate cortex of man. Hum Neurobiol 6(1):1–9
Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387(2):167–178
Huttenlocher PR, de Court, Garey LJ, Van der LH (1982a) Synaptic development in human cerebral cortex. Int J Neurol 16–17:144–154
Huttenlocher PR, de Court, Garey LJ, Van der LH (1982b) Synaptogenesis in human visual cortex–evidence for synapse elimination during normal development. Neurosci Lett 33(3):247–252
Julesz B (1986) Texton gradients: the texton theory revisited. Biol Cybern 54(4–5):245–251
Julesz B, Bergen JR (1983) Textons, the fundamental elements in preattentive vision and perception of textures. Bell Syst Tech J 62:1619–1645
Kastner S, De WP, Ungerleider LG (2000) Texture segregation in the human visual cortex: a functional MRI study. J Neurophysiol 83(4):2453–2457
Kovacs I (2000) Human development of perceptual organization. Vision Res 40(10–12):1301–1310
Lachapelle J, Ouimet C, Bach M, Ptito A, McKerral M (2004) Texture segregation in traumatic brain injury–a VEP study. Vision Res 44(24):2835–2842
Lamme VA, van Dijk BW, Spekreijse H (1992) Texture segregation is processed by primary visual cortex in man and monkey. Evidence from VEP experiments. Vision Res 32(5):797–807
Lippe S, Roy MS, Perchet C, Lassonde M (2006) Electrophysiological markers of visuocortical development. Cereb Cortex. Advance Access published February 8, 2006. doi:10.1093/cercor/bhj130
Luck SJ (2005) An introduction to the event-related potential technique. The MIT Press, Cambridge p 376
Meigen T, Bach M (1999) Texture-segregation specific VEP component can be modulated by an additional task. Invest Ophthalmol Vis Sci 40:S823
Moskowitz A, Sokol S (1983) Developmental changes in the human visual system as reflected by the latency of the pattern reversal VEP. Electroencephalogr Clin Neurophysiol 56(1):1–15
Norcia AM, Pei F, Bonneh Y, Hou C, Sampath V, Pettet MW (2005) Development of sensitivity to texture and contour information in the human infant. J Cogn Neurosci 17(4):569–579
Nothdurft HC (1990) Texton segregation by associated differences in global and local luminance distribution. In: Proceedings of the Royal Society of London 239:295–320
Nothdurft HC (2000) Salience from feature contrast: temporal properties of saliency mechanisms. Vision Res 40:2421–2435
Ponton CW, Eggermont JJ, Kwong B, Don M (2000) Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol 111(2):220–236
Rieth C, Sireteanu R (1994) Texture segmentation and ‘pop-out’ in infants and children: the effect of test field size. Spat Vis 8(2):173–191
Roy MS, Barsoum-Homsy M, Orquin J, Benoit J (1995) Maturation of binocular pattern visual evoked potentials in normal full-term and preterm infants from 1 to 6 months of age. Pediatr Res 37(2):140–144
Sampaio RC, Truwit CL (2005) Myelination in the developing Human brain. In: Nelson CA, Luciana M (eds) Handbook of developmental cognititve neuroscience. The MIT Press, Cambridge pp 35–44
Sireteanu R, Rieth C (1992) Texture segregation in infants and children. Behav Brain Res 49(1):133–139
Treisman A (1985) Preattentive processing in vision. Comput Vis Graph Image Process 31:156–177
Tsuneishi S, Casaer P (1997) Stepwise decrease in VEP latencies and the process of myelination in the human visual pathway. Brain Dev 19(8):547–551
Tucker DM (1993) Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalogr Clin Neurophysiol 87(3):154–163
Vaughan HG, Kurtzberg D (1992) Electrophysiological indices of human brain maturation and cognitive development. In: Gunnar MR, Nelson CA, (eds). Minnesota Symposia on Child Psychology, Erlbaum, Hillsdale p 1–36
Yakovlev PI, Lecours AR (1967) The myelogenetic cycles of regional maturation of the brain. In: Minkowski A (ed) Regional development of the brain in early life. Blackwell, Oxford pp 3–20
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (MOP-62888 awarded to M.-S. R., M. L. and M. M.), the Canadian Foundation for Innovation (awarded to M.L.) and the Natural Sciences and Engineering Research Council (awarded to F.L.), by a ‘chercheur-boursier clinicien’ salary award to M.M. and a scholarship awarded to C.A. both from the ‘Fonds de la Recherche en Santé du Québec’, as well as by a scholarship to C.A. by the ‘Fonds de Recherche en Ophtalmologie de l’Université de Montréal’. The authors wish to thank Prof. Michael Bach, Universitäts-Augenklinik Freiburg, Germany, and Miss Julie Lachapelle, pht., for their support in adapting the tsVEP technique for developmental studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arcand, C., Tremblay, E., Vannasing, P. et al. Development of visual texture segregation during the first year of life: a high-density electrophysiological study. Exp Brain Res 180, 263–272 (2007). https://doi.org/10.1007/s00221-007-0854-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-0854-y