Abstract
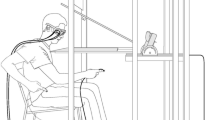
Successful adaptation to novel sensorimotor contexts critically depends on efficient sensory processing and integration mechanisms, particularly those required to combine visual and proprioceptive inputs. If the basal ganglia are a critical part of specialized circuits that adapt motor behavior to new sensorimotor contexts, then patients who are suffering from basal ganglia dysfunction, as in Parkinson’s disease should show sensorimotor learning impairments. However, this issue has been under-explored. We tested the ability of 8 patients with Parkinson’s disease (PD), off medication, ten healthy elderly subjects and ten healthy young adults to reach to a remembered 3D location presented in an immersive virtual environment. A multi-phase learning paradigm was used having four conditions: baseline, initial learning, reversal learning and aftereffect. In initial learning, the computer altered the position of a simulated arm endpoint used for movement feedback by shifting its apparent location diagonally, requiring thereby both horizontal and vertical compensations. This visual distortion forced subjects to learn new coordinations between what they saw in the virtual environment and the actual position of their limbs, which they had to derive from proprioceptive information (or efference copy). In reversal learning, the sign of the distortion was reversed. Both elderly subjects and PD patients showed learning phase-dependent difficulties. First, elderly controls were slower than young subjects when learning both dimensions of the initial biaxial discordance. However, their performance improved during reversal learning and as a result elderly and young controls showed similar adaptation rates during reversal learning. Second, in striking contrast to healthy elderly subjects, PD patients were more profoundly impaired during the reversal phase of learning. PD patients were able to learn the initial biaxial discordance but were on average slower than age-matched controls in adapting to the horizontal component of the biaxial discordance. More importantly, when the biaxial discordance was reversed, PD patients were unable to make appropriate movement corrections. Therefore, they showed significantly degraded learning indices relative to age-matched controls for both dimensions of the biaxial discordance. Together, these results suggest that the ability to adapt to a sudden biaxial visuomotor discordance applied in three-dimensional space declines in normal aging and Parkinson disease. Furthermore, the presence of learning rate differences in the PD patients relative to age-matched controls supports an important contribution of basal ganglia-related circuits in learning novel visuomotor coordinations, particularly those in which subjects must learn to adapt to sensorimotor contingencies that were reversed from those just learned.



Similar content being viewed by others
Notes
An eleventh elderly subject was run, but his data were excluded since analysis revealed that his spatial errors fell well outside those of all other elderly control subjects; they were greater than two standard deviations from the mean
The number of trials tested is different for the initial learning (20 trials) and the reversal learning phases (15 trials). To compare more directly performance indices between the two learning phases, we also performed statistical analyses on the learning phases using only the first 15 trials. For instance, we evaluated the variability indexes and the adaptation magnitude scores on trials 10–15 of initial learning as was done for reversal learning. Similar results were obtained as when using all 20 trials of initial learning. The adaptation score for the horizontal component of the initial biaxial discordance was similar in all three subject groups (F (2,25) = 0.285; P > 0.05) as was the level of trial-to-trial variability (F (2,25) = 0.012; P > 0.05). Also, there was a significant between group difference in the adaptation magnitude score for the vertical component of the initial discordance (F (2,25) = 8.49; P < 0.05). Young and elderly controls showed a similar level of adaptation (P > 0.05), while PD patients showed significantly smaller adaptation magnitude score (P < 0.025). In a similar manner, we performed the ANOVAs on constant errors for trials 1–15 of initial learning. Again, these analyses confirmed results found when trials 1–20 were analyzed
References
Adamovich SV, Berkinblit MB, Henning W, Sage J, Poizner H. (2001) The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience 1027–1041
Alexander GE (1994) Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol 11:420–431
Azulay JP, Mesure S, Amblard B, Pouget J (2002) Increased visual dependence in Parkinson’s disease. Percept Mot Skills 95(3 Pt 2):1106–1114
Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM (2005) Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437(7062):1158–1161
Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD (1986) Performance of simultaneous movements in patients with Parkinson’s disease. Brain Aug 109(Pt 4):739–57
Berns SB, Sejnowski TJ (1998) A computational model of how the basal ganglia produce sequences. J Cogn Neurosci 10:108–121
Bock O, Schneider S (2001) Acquisition of a sensorimotor skill in younger and older adults. Acta Physiol Pharmacol Bulg 26(1–2):89–92
Bock O, Schneider S (2002) Sensorimotor adaptation in young and elderly humans. Neurosci Biobehav Rev 26:761–767
Bronstein AM, Yardley L, Moore AP, Cleeves L (1996) Visually and posturally mediated tilt illusion in Parkinson's disease and in labyrinthine defective subjects. Neurology 47(3):651–656
Brooks DJ (2000) Imaging basal ganglia function. J Anat 196:543–554
Buch ER, Young S, Contreras-Vidal JL (2003) Visuomotor adaptation in normal aging. Learn Mem 10:55–63
Contreras-Vidal JL, Buch ER (2003) Effects of Parkinson’s deasese on visuomotor adaptation. Exp Brain Res 150:25–32
Contreras-Vidal JL, Gold DR (2004) Dynamic estimation of hand position is abnormal in Parkinson’s disease. Parkinsonism Relat Disord 10(8):501–506
Contreras-Vidal JL, Teulings HL, Stelmach GE, Adler CH (2002) Adaptation to changes in vertical display gain during handwriting in Parkinson’s disease patients, elderly and young controls. Parkinsonism Relat Disord 9:77–84
Cools AR, Van Den Bercken JH, Horstink MW, Van Spaendonck KP, Berger HJ (1984) Cognitive and motor shifting aptitude disorder in Parkinson’s disease. J Neurol Neurosurg Psychiatry 47(5):443–453
Cronin-Golomb A, Corkin S, Growdon JH (1994) Impaired problem solving in Parkinson’s disease: impact of a set- shifting deficit. Neuropsychologia 32:579–593
Desmurget M, Jordan M, Prablanc C, Jeannerod M (1997) Constrained and unconstrained movements involve different control strategies. J Neurophysiol Mar 77(3):1644–1650
Desmurget M, Gaveau P, Vindras RS, Turner E, Broussolle S Thobois (2004) On-line motor control in patients with Parkinson’s disease. Brain 127(Pt 8):1755–1773 Epub Jun 23
Doyon J, Penhune V, Ungerleider LG (2003) Distinct contribution of the cortico-strial and cortico cerebellar systems to motor skill learning. Neuropsychologia 41:252–262
Ebersbach G, Hattig H, Schelosky L, Wissel J, Poewe W (1994) Perseverative motor behaviour in Parkinson’s disease. Neuropsychologia 32(7):799–804
Fahn S, Elton RL (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden C, Calne D Goldstein M (eds) Recent developments in Parkinson’s disease. Macmillan:29304, New York
Fernandez-Ruiz J, Hall C, Vergara P, Diaz R (2000) Prism adaptation in normal aging: Slower adaptation rate and larger after effect. Brain Res Cogn Brain Res 9:223–226
Fernandez-Ruiz J, Diaz R, Hall-Haro C, Vergara P, Mischner J, Nunez L, Drucker-Colin R, Ochoa A, Alonso ME (2003) Normal prism adaptation but reduced after-effect in basal ganglia disorders using a throwing task 18:689–694
Florian A, Kagerer, Contreras-Vidal JL, Stelmach GE (1997) Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res Jul 115(3):557–561
Flowers K (1976) Visual “closed-loop” and “open-loop” characteristics of voluntary movement in patients with parkinsonism and intention tremor. Brain 99:269–310
Fucetola R, Smith MC (1997) Distorted visual feedback effects on drawing in Parkinson’s disease. Acta Psychol (Amst) 95:255–266
Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F (2000) Visual feedback has differential effects on reaching movements in Parkinson’s and Alzheimer’s disease. Brain Res 876:112–113
Grafton ST, Hazeltine E, Ivry R (1995) Functional mapping of sequence learning in normal humans. J Cogn Neurosci 7:497–510
Graybiel AM (2004) Network-level neuroplasticiy in cortico-basal ganglia pathways, Parkinsonism Rel Disord 10:293–296
Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 15(6):638–644
Graydon FX, Friston KJ, Thomas CG, Brooks VB, Menon RS (2005) Learning-related fMRI activation associated with a rotational visuo-motor transformation. Cogn Brain Res 22:373–383
Haaland KY, Harrington DL, O’Brien S, Hermanowicz N (1997) Cognitive-motor learning in Parkinson’s disease. Neuropsychology 11:180–186
Hayes AE, Davidson MC, Keele SW, Rafal RD (1998) Toward a functional analysis of the basal ganglia. J Cogn Neurosci 10:178–198
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Hoover JE, Strick PL (1993) Multiple output channels in the basal ganglia. Science 259:819–821
Houk JC, Wise SP (1995) Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex 5:95–110
Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B et al (2000) Human cerellar activity reflecting an acquired internal model of a new tool. Nature 403:192–195
Inoue K, Kawashima R, Satoh K, Kinomura S, Sugiura M, Goto R, Ito M, Fukuda H (2000) A PET study of visuomotor learning under optical rotation. Neuroimage 11:505–516
Jackson GM, Jackson SR, Hindle JV (2000) The control of bimanual reach-to-grasp movements in hemiparkinsonian patients. Exp Brain Res 132:390–398
Jobst EE, Melnick ME, Byl NN, Dowling GA, Aminoff MJ (1997) Sensory perception in Parkinson disease. Arch Neurol 54:450–454
Jueptner M, Jenkins IH, Brooks DJ, Frackowiak RS, Passingham RE (1996) The sensory guidance of movement: a comparison of the cerebellum and basal ganglia. Exp Brain Res 112:462–474
Keijsers NL, Admiraal MA, Cools AR, Bloem BR, Gielen CC (2005) Differential progression of proprioceptive and visual information processing deficits in Parkinson’s disease. Eur J Neurosci 21(1):239–248
Klockgether T, Dichgans J (1994) Visual control of arm movements in Parkinson's disease. Mov Disord 9(1):48–56
Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J (1995) A defect of kinesthesia in Parkinson’s disease. Mov Disord 10:460–465
Krakauer JW, Ghilardi MD, Mentis M, Barnes A, Veytsman, Eidelberg D, Ghez C (2004) Differential cortical and subcortical activations in learning rotations and gains for reaching: a pet study. J Neurophysiol 91:924–933
Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH, Fischman AJ, Alpert NM (1998) Robot aided functional imaging: application to a motor learning study. Hum Brain Mapp 6:59–72
Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H (2001) Procedural motor learning in Parkinson’s disease. Exp Brain Res 141:425–437
Kropotow JD, Etlinger SC (1999) Selection of actions in the basal ganglia-thalamocortical circuits: review and model. Int J Psychophysiol 31:197–217
Lee AC, Harris JP, Atkinson EA, Fowler MS (2001) Evidence from a line bisection task for visuospatial neglect in Left Hemiparkinson’s disease. Vis Res 41:2677–2686
Lee AC, Harris JP, Atkinson EA, Nithi K, Fowler MS (2002) Dopamine and the representation of the upper visual field: evidence from vertical bisection errors in unilateral Parkinson’s disease. Neuropsychologia 40:2023–2029
Malfait N, Ostry DJ (2004) Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24(37):8084–8089
Maschke M, Gomez CM, Tuite PJ, Konczak J (2003) Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 126:2312–2322
Messier J, Kalaska JF (1997) Differential effect of task conditions on errors of direction and extent of reaching movements. Exp Brain Res 115:469–478
Monchi O, Petrides M, Doyon J, Postuma RV, Worsley K, Dagher A (2004) Neural bases of set-Shifting deficits in Parkinson’s disease. J Neurosci 24(3):702–710
Pessiglione M, Guehl D, Agid Y, Hirsch EC, Feger J, Tremblay L (2003) Impairment of context-adapted movement selection in a primate model of presymptomatic Parkinson’s disease. Brain 126(Pt 6):1392–1408
Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L (2005) Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci 25(6):1523–1531
Poizner H, Fookson O, Berkinglit MB, Hening W, Feldman G, Adamovich SV (1998) Pointing to remembered targets in 3D space in Parkinson’s disease. Motor Control 2:251–277
Poizner H, Feldman AG, Levin MF, Berkinblit MB, Hening WA, Patel A, Adamovich SV (2000) The timing of arm-trunk coordination is deficient and vision-dependent in Parkinson’s patients during reaching movements. Exp Brain Res 133(3):279–292
Proctor F, Riklan M, Cooper IS, Teuber H-L (1964) Judgment of visual and postural vertical by Parkinsonian patients. Neurology 14:282–293
Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD et al (1997) Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Mapp 5:124–132
Richards M, Cote LJ, Stern Y (1993) Executive function in Parkinson’s Disease: set-shifting or set-maintenance? J Clin Exp Neuropsychol 15 2:266–279
Robertson EM, Miall RC (1999) Visuomotor adaptation during inactivation of the dentate nucleus; NeuroReport 10(5):1029–1034
Schmidt WJ (1998) Dopamine-glutamate interactions in the basal ganglia. Amino Acids 14:5–10
Schneider JS, Diamond SG, Markham CH (1986) Deficits in orofacial sensorimotor function in Parkinson’s disease. Ann Neurol 19:275–282
Seidler RD, Noll DC, Chintalapati P (2006) Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res [Epub ahead of print]
Shadmehr R, Holcomb HH (1999) Inhibitory control of competing motor memories. Exp Brain Res 126:235–251
Stern Y, Mayeux R, Hermann A, Rosen J (1988) Prism adaptation in Parkinson’s disease. J Neurol Neuroseurg Psychiatry 51:1584–1587
Stoffers D, Berendse HW, Deijen JB, Wolters EC (2001) Motor perseveration is an early sign of Parkinson’s disease. Neurology 57:2111–2113
Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH (2002) Adaptation of handwriting size under distorted visual feedback in patients witn parkinson’s disease and elderly and young controls. J Neurol Neurosurg Psychiatry 72:315–324
Vakil E, Herishanu-Naaman S (1998) Declarative and procedural learning in Parkinson’s disease patients having tremor or bradykinesia as the predominant symptom. Cortex 34:611–620
Zia S, Cody F, O’Boyle D (2000) Joint position sense is impaired by Parkinson’s disease. Ann Neurol 47:218–228
Acknowledgments
This research was supported in part by an FRSQ and CIHR Postdoctoral Fellowship to JM and by NIH Grant 7 R01 NS36449 to UCSD (HP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Messier, J., Adamovich, S., Jack, D. et al. Visuomotor learning in immersive 3D virtual reality in Parkinson’s disease and in aging. Exp Brain Res 179, 457–474 (2007). https://doi.org/10.1007/s00221-006-0802-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0802-2