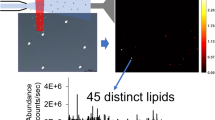
Abstract
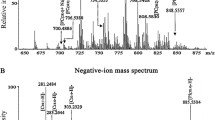
In breast cancer, overexpression of human epidermal growth factor receptor 2 (HER2) correlates with overactivation of lipogenesis, mutation of tumor suppressor p53, and increased metastatic potential. The mechanisms through which lipids mediate p53, HER2, and metastatic potential are largely unknown. We have developed a desorption electrospray ionization mass spectrometry (DESI-MS) method to identify lipid biomarkers of HER2/p53 expression, metastatic potential, and disease state (viz. cancer vs. non-cancerous) in monolayer and suspension breast cancer cell cultures (metastatic potential: MCF-7, T-47D, MDA-MB-231; HER2/p53: HCC2218 (HER2+++/p53+), HCC1599 (HER2−/p53−), HCC202 (HER2++/p53−), HCC1419 (HER2+++/p53−) HCC70 (HER2−/p53+++); non-cancerous: MCF-10A). Unsupervised principal component analysis (PCA) of DESI-MS spectra enabled identification of twelve lipid biomarkers of metastatic potential and disease state, as well as ten lipids that distinguish cell lines based on HER2/p53 expression levels (> 200 lipids were identified per cell line). In addition, we developed a DESI-MS imaging (DESI-MSI) method for mapping the spatial distribution of lipids in metastatic spheroids (MDA-MB-231). Of the twelve lipids that correlate with changes in the metastatic potential of monolayer cell cultures, three were localized to the necrotic core of spheroids, indicating a potential role in promoting cancer cell survival in nutrient-deficient environments. One lipid species, which was not detected in monolayer MDA-MB-231 cultures, was spatially localized to the periphery of the spheroid, suggesting a potential role in invasion and/or proliferation. These results demonstrate that combining DESI-MS/PCA of monolayer and suspension cell cultures with DESI-MSI of spheroids is a promising approach for identifying lipid biomarkers of specific genotypes and phenotypes, as well as elucidating the potential function of these biomarkers in breast cancer.

Graphical Absract





Similar content being viewed by others
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Hilvo M, Denkert C, Lehtinen L, Mueller B, Brockmoeller S, Seppanen-Laakso T, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71(9):3236–45.
Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77.
Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70(20):8117–26.
Baumann J, Sevinsky C, Conklin DS. Lipid biology of breast cancer. Biochim Biophys Acta. 2013;1831(10):1509–17.
Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66(10):5287–94.
Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67(17):8180–7.
Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65(15):6719–25.
De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63(13):3799–804.
Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–510.
Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70.
Brunet J, Vazquez-Martin A, Colomer R, Grana-Suarez B, Martin-Castillo B, Menendez JA. BRCA1 and acetyl-CoA carboxylase: the metabolic syndrome of breast cancer. Mol Carcinog. 2008;47(2):157–63.
Moreau K, Dizin E, Ray H, Luquain C, Lefai E, Foufelle F, et al. BRCA1 affects lipid synthesis through its interaction with acetyl-CoA carboxylase. J Biol Chem. 2006;281(6):3172–81.
Menendez JA. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: molecular mechanisms and therapeutic perspectives. Biochim Biophys Acta. 2010;1801(3):381–91.
Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007;67(3):1262–9.
Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64(6):2070–5.
Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311(5767):1566–70.
Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–3.
Guenther S, Muirhead LJ, Speller AVM, Golf O, Strittmatter N, Ramakrishnan R, et al. Spatially resolved metabolic phenotyping of breast cancer by desorption electrospray ionization mass spectrometry. Cancer Res. 2015;75(9):1828–37.
Porcari AM, Zhang JL, Garza KY, Rodrigues-Peres RM, Lin JQ, Young JH, et al. Multicenter study using desorption-electrospray-ionization-mass-spectrometry imaging for breast-cancer diagnosis. Anal Chem. 2018;90(19):11324–32.
Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10.
Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381–94.
Jayashree B, Srimany A, Jayaraman S, Bhutra A, Janakiraman N, Chitipothu S, et al. Monitoring of changes in lipid profiles during PLK1 knockdown in cancer cells using DESI MS. Anal Bioanal Chem. 2016;408(20):5623–32.
Perry RH, Bellovin DI, Shroff EH, Ismail AI, Zabuawala T, Felsher DW, et al. Characterization of MYC-induced tumorigenesis by in situ lipid profiling. Anal Chem. 2013;85(9):4259–62.
Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro - a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15(5):405–12.
Li HH, Hummon AB. Imaging mass spectrometry of three-dimensional cell culture systems. Anal Chem. 2011;83(22):8794–801.
Weaver EM, Hummon AB. Imaging mass spectrometry: from tissue sections to cell cultures. Adv Drug Deliv Rev. 2013;65(8):1039–55.
Liu X, Weaver EM, Hummon AB. Evaluation of therapeutics in three-dimensional cell culture systems by MALDI imaging mass spectrometry. Anal Chem. 2013;85(13):6295–302.
Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–92.
Liu X, Lukowski JK, Flinders C, Kim S, Georgiadis RA, Mumenthaler SM, et al. MALDI-MSI of immunotherapy: mapping the EGFR-targeting antibody cetuximab in 3D colon-cancer cell cultures. Anal Chem. 2018;90(24):14156–64.
Perry RH, Cooks RG, Noll RJ. Orbitrap mass spectrometry: instrumentation, ion motion, and applications. Mass Spectrom Rev. 2008;27(6):661–99.
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4(3):309–24.
Gazdar AF, Kurvari V, Virmani A, Gollahon L, Sakaguchi M, Westerfield M, et al. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int J Cancer. 1998;78(6):766–74.
Doria ML, Cotrim Z, Macedo B, Simoes C, Domingues P, Helguero L, et al. Lipidomic approach to identify patterns in phospholipid profiles and define class differences in mammary epithelial and breast cancer cells. Breast Cancer Res Treat. 2012;133(2):635–48.
Ziegler E, Hansen MT, Haase M, Emons G, Grundker C. Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Res Treat. 2014;148(2):269–77.
Masters JRW. Animal cell culture. Oxford: Oxford University Press; 2000.
Comi TJ, Ryu SW, Perry RH. Synchronized desorption electrospray ionization mass spectrometry imaging. Anal Chem. 2015;88:1169–75.
Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30(10):918–20.
Menon SS, Guruvayoorappan C, Sakthivel KM, Rasmi RR. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. 2019;491:39–45.
Kanyilmaz G, Yavuz BB, Aktan M, Karaagac M, Uyar M, Findik S. Prognostic importance of Ki-67 in breast cancer and its relationship with other prognostic factors. Eur J Breast Health. 2019;15(4):256–61.
Doria ML, Cotrim CZ, Simoes C, Macedo B, Domingues P, Domingues MR, et al. Lipidomic analysis of phospholipids from human mammary epithelial and breast cancer cell lines. J Cell Physiol. 2013;228(2):457–68.
Messias MCF, Mecatti GC, Priolli DG, Carvalho PD. Plasmalogen lipids: functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018;17.
Sharma B, Kanwar SS. Phosphatidylserine: a cancer cell targeting biomarker. Semin Cancer Biol. 2018;52:17–25.
Krishnamoorthy S, Honn KV. Eicosanoids and other lipid mediators and the tumor hypoxic microenvironment. Cancer Metastasis Rev. 2011;30(3–4):613–8.
Mylonis I, Simos G, Paraskeva E. Hypoxia-inducible factors and the regulation of lipid metabolism. Cells. 2019;8(3).
Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5.
Patwardhan GA, Liu Y-Y. Sphingolipids and expression regulation of genes in cancer. Prog Lipid Res. 2011;50(1):104–14.
Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7(4):281–94.
Omori T, Honda A, Mihara H, Kurihara T, Esaki N. Identification of novel mammalian phospholipids containing threonine, aspartate, and glutamate as the base moiety. J Chromatogr B. 2011;879(29):3296–302.
Vara JAF, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501.
Acknowledgments
The authors wish to acknowledge Nova Southeastern University (NSU) and the University of Illinois at Urbana-Champaign (UIUC) for financial support (all experiments were performed at UIUC). We would also thank Dr. Sandy McMasters of the UIUC Cell Media Facility. We also acknowledge the assistance of Chethani Chitraacharige (NSU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1.13 mb)
Rights and permissions
About this article
Cite this article
Robison, H.M., Chini, C.E., Comi, T.J. et al. Identification of lipid biomarkers of metastatic potential and gene expression (HER2/p53) in human breast cancer cell cultures using ambient mass spectrometry. Anal Bioanal Chem 412, 2949–2961 (2020). https://doi.org/10.1007/s00216-020-02537-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02537-4