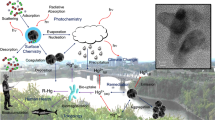
Abstract
The phase of oxidized mercury is critical in the fate, transformation, and bioavailability of mercury species in Earth’s ecosystem. There is now evidence that what is measured as gaseous oxidized mercury (GOM) is not only gaseous but also consists of airborne nanoparticles with distinct physicochemical properties. Herein, we present the development of the first method for the consistent and reproducible generation of oxidized mercury nano- and sub-micron particles (~ 5 to 400 nm). Oxidized mercury nanoparticles are generated using two methods, vapor-phase condensation and aqueous nebulization, for three proxies: mercury(II) bromide (HgBr2), mercury(II) chloride (HgCl2), and mercury(II) oxide (HgO). These aerosols are characterized using scanning mobility and optical sizing, high-resolution scanning transmission electron microscopy (STEM), and nano/microparticle interface coupled to soft ionization mercury mass spectrometric techniques. Synthetic nanoparticle stability was studied in aqueous media, and using a microcosm at ambient tropospheric conditions of ~ 740 Torr pressure, room temperature, and at relative humidity of approximately 20%. Analysis of microcosm airborne nanoparticles confirmed that generated synthetic mercury nanoparticles retain their physical properties once in air. KCl-coated denuders, which are currently used globally to measure gaseous mercury compounds, were exposed to generated oxidized mercury nanoparticles. The degree of synthetic mercury nanoparticle capture by KCl-coated denuders and particulate filters was assessed. A significant portion of nanoparticulate and sub-micron particulate mercury was trapped on the KCl-coated denuder and measured as GOM. Finally, we demonstrate the applicability of soft ionization mercury mass spectrometry to the measurement of mercury species present in the gaseous and solid phase. We recommend coupling of this technique with existing methodology for a more accurate representation of mercury biogeochemistry cycling.

Graphical Abstract








Similar content being viewed by others
Change history
16 January 2020
The authors would like to call the reader’s attention to the fact that, unfortunately, there was an unintentional oversight regarding the funding information in this manuscript; please find the correct information below.
16 January 2020
The authors would like to call the reader���s attention to the fact that, unfortunately, there was an unintentional oversight regarding the funding information in this manuscript; please find the correct information below.
References
Malcolm EG, Ford AC, Redding TA, Richardson MC, Strain BM, Tetzner SW. Experimental investigation of the scavenging of gaseous mercury by sea salt aerosol. J Atmos Chem. 2010;63(3):221–34. https://doi.org/10.1007/s10874-010-9165-y.
Denis MS, Song X, Lu JY, Feng X. Atmospheric gaseous elemental mercury in downtown Toronto. Atmos Environ. 2006;40(21):4016–24. https://doi.org/10.1016/j.atmosenv.2005.07.078.
Cairns E, Tharumakulasingam K, Athar M, Yousaf M, Cheng I, Huang Y, et al. Source, concentration, and distribution of elemental mercury in the atmosphere in Toronto, Canada. Environ Pollut. 2011;159(8–9):2003–8. https://doi.org/10.1016/j.envpol.2010.12.006.
Deeds DA, Ghoshdastidar A, Raofie F, Guerette EA, Tessier A, Ariya PA. Development of a particle-trap preconcentration-soft ionization mass spectrometric technique for the quantification of mercury halides in air. Anal Chem. 2015;87(10):5109–16. https://doi.org/10.1021/ac504545w.
Seo Y-S, Han Y-J, Choi H-D, Holsen TM, Yi S-M. Characteristics of total mercury (TM) wet deposition: scavenging of atmospheric mercury species. Atmos Environ. 2012;49:69–76. https://doi.org/10.1016/j.atmosenv.2011.12.031.
Xiao Z, Sommar J, Wei S, Lindqvist O. Sampling and determination of gas phase divalent mercury in the air using a KCl coated denuder. Fresen J Anal Chem. 1997;358(3):386–91. https://doi.org/10.1007/s002160050434.
McClure CD, Jaffe DA, Edgerton ES. Evaluation of the KCl denuder method for gaseous oxidized mercury using HgBr2 at an in-service AMNet site. Environ Sci Technol. 2014;48(19):11437–44. https://doi.org/10.1021/es502545k.
Lyman SN, Jaffe DA, Gustin MS. Release of mercury halides from KCl denuders in the presence of ozone. Atmos Chem Phys. 2010;10(17):8197–204. https://doi.org/10.5194/acp-10-8197-2010.
Huang JY, Miller MB, Weiss-Penzias P, Gustin MS. Comparison of gaseous oxidized Hg measured by KCl-coated denuders, and nylon and cation exchange membranes. Environ Sci Technol. 2013;47(13):7307–16. https://doi.org/10.1021/es4012349.
Landis MS, Stevens RK, Schaedlich F, Prestbo EM. Development and characterization of an annular denuder methodology for the measurement of divalent inorganic reactive gaseous mercury in ambient air. Environ Sci Technol. 2002;36(13):3000–9. https://doi.org/10.1021/es015887t.
Lynam MM, Keeler GJ. Artifacts associated with the measurement of particulate mercury in an urban environment: the influence of elevated ozone concentrations. Atmos Environ. 2005;39(17):3081–8. https://doi.org/10.1016/j.atmosenv.2005.01.036.
Pal B, Ariya PA. Studies of ozone initiated reactions of gaseous mercury: kinetics, product studies, and atmospheric implications. Phys Chem Chem Phys. 2004;6(3):572–9.
Pal B, Ariya PA. Gas-phase HO•-initiated reactions of elemental mercury: kinetics, product studies, and atmospheric implications. Environ Sci Technol. 2004;38(21):5555–66. https://doi.org/10.1021/es0494353.
Ariya PA, Khalizov A, Gidas A. Reactions of gaseous mercury with atomic and molecular halogens: kinetics, product studies, and atmospheric implications. J Phys Chem A. 2002;106(32):7310–20. https://doi.org/10.1021/jp020719o.
Gustin M, Jaffe D. Reducing the uncertainty in measurement and understanding of mercury in the atmosphere. Environ Sci Technol. 2010;44(7):2222–7. https://doi.org/10.1021/es902736k.
Yang H (2002) Effects of fly ash on the oxidation of mercury during post-combustion conditions.
Kos G, Ryzhkov A, Dastoor A, Narayan J, Steffen A, Ariya PA, et al. Evaluation of discrepancy between measured and modelled oxidized mercury species. Atmos Chem Phys. 2013;13(9):4839–63. https://doi.org/10.5194/acp-13-4839-2013.
Raofie F, Ariya PA. Product study of the gas-phase BrO-initiated oxidation of Hg0: evidence for stable Hg1+ compounds. Environ Sci Technol. 2004;38(16):4319–26.
Feng XB, Lu JY, Gregoire DC, Hao YJ, Banic CM, Schroeder WH. Analysis of inorganic mercury species associated with airborne particulate matter/aerosols: method development. Anal Bioanal Chem. 2004;380(4):683–9. https://doi.org/10.1007/s00216-004-2803-y.
Pyta H, Rogula-Kozlowska W. Determination of mercury in size-segregated ambient particulate matter using CVAAS. Microchem J. 2016;124:76–81. https://doi.org/10.1016/j.microc.2015.08.001.
Malcolm EG, Keeler GJ. Evidence for a sampling artifact for particulate-phase mercury in the marine atmosphere. Atmos Environ. 2007;41(16):3352–9. https://doi.org/10.1016/j.atmosenv.2006.12.024.
Murphy DM, Thomson DS, Mahoney MJ. In situ measurements of organics, meteoritic material, mercury, and other elements in aerosols at 5 to 19 kilometers. Science. 1998;282(5394):1664–9. https://doi.org/10.1126/science.282.5394.1664.
Marusczak N, Sonke JE, Fu X, Jiskra M. Tropospheric GOM at the pic du Midi observatory-correcting bias in denuder based observations. Environ Sci Technol. 2016. https://doi.org/10.1021/acs.est.6b04999.
Nazarenko Y, Rangel-Alvarado RB, Kos G, Kurien U, Ariya PA. Novel aerosol analysis approach for characterization of nanoparticulate matter in snow. Environ Sci Pollut Res. 2016:1–14. https://doi.org/10.1007/s11356-016-8199-3.
Mohadesi A, Ranjbar M, Hosseinpour-Mashkani SM. Solvent-free synthesis of mercury oxide nanoparticles by a simple thermal decomposition method. Superlattice Microst. 2014;66:48–53. https://doi.org/10.1016/j.spmi.2013.11.017.
Liu J, Pui DYH, Wang J. Removal of airborne nanoparticles by membrane coated filters. Sci Total Environ. 2011;409(22):4868–74. https://doi.org/10.1016/j.scitotenv.2011.08.011.
Ghoshdastidar AJ, Ariya PA. The existence of airborne mercury nanoparticles. Sci Rep-Uk. 2019;9(1):10733. https://doi.org/10.1038/s41598-019-47086-8.
Jen YH, Chen WH, Yuan CS, Ie IR, Hung CH. Seasonal variation and spatial distribution of atmospheric mercury and its gas-particulate partition in the vicinity of a semiconductor manufacturing complex. Environ Sci Pollut Res. 2014;21(8):5474–83. https://doi.org/10.1007/s11356-013-2441-z.
Subir M, Ariya PA, Dastoor AP. A review of the sources of uncertainties in atmospheric mercury modeling II. Mercury surface and heterogeneous chemistry - a missing link. Atmos Environ. 2012;46:1–10. https://doi.org/10.1016/j.atmosenv.2011.07.047.
Seinfeld JH, Pandis SN. Atmospheric chemistry and physics: from air pollution to climate change: John Wiley & Sons; 2012.
Grassian VH. When size really matters: size-dependent properties and surface chemistry of metal and metal oxide nanoparticles in gas and liquid phase environments†. J Phys Chem C. 2008;112(47):18303–13. https://doi.org/10.1021/jp806073t.
Murphy DM, Hudson PK, Thomson DS, Sheridan PJ, Wilson JC. Observations of mercury-containing aerosols. Environ Sci Technol. 2006;40(10):3163–7. https://doi.org/10.1021/es052385x.
Si L, Ariya PA. Photochemical reactions of divalent mercury with thioglycolic acid: formation of mercuric sulfide particles. Chemosphere. 2015;119:467–72. https://doi.org/10.1016/j.chemosphere.2014.07.022.
Pham AL-T, Morris A, Zhang T, Ticknor J, Levard C, Hsu-Kim H. Precipitation of nanoscale mercuric sulfides in the presence of natural organic matter: structural properties, aggregation, and biotransformation. Geochim Cosmochim Acta. 2014;133(0):204–15. https://doi.org/10.1016/j.gca.2014.02.027.
Ariya PA, Amyot M, Dastoor A, Deeds D, Feinberg A, Kos G, et al. Mercury physicochemical and biogeochemical transformation in the atmosphere and at atmospheric interfaces: a review and future directions. Chem Rev. 2015;115(10):3760–802. https://doi.org/10.1021/cr500667e.
Mwilu SK, El Badawy AM, Bradham K, Nelson C, Thomas D, Scheckel KG, et al. Changes in silver nanoparticles exposed to human synthetic stomach fluid: effects of particle size and surface chemistry. Sci Total Environ. 2013;447(0):90–8. https://doi.org/10.1016/j.scitotenv.2012.12.036.
Mazrui Nashaat M, Seelen E, King'ondu CK, Thota S, Awino J, Rouge J, et al. The precipitation, growth and stability of mercury sulfide nanoparticles formed in the presence of marine dissolved organic matter. Environ Sci Process Impacts. 2018;20(4):642–56. https://doi.org/10.1039/C7EM00593H.
Sinha A, Khare SK. Mercury bioaccumulation and simultaneous nanoparticle synthesis by Enterobacter sp. cells. Bioresour Technol. 2011;102(5):4281–4. https://doi.org/10.1016/j.biortech.2010.12.040.
Clever HL, Johnson SA, Derrick ME. The solubility of mercury and some sparingly soluble mercury salts in water and aqueous electrolyte solutions. J Phys Chem Ref Data. 1985;14(3):631–80. https://doi.org/10.1063/1.555732.
Acknowledgments
The authors thank Dr. Hojatollah Vali and David Liu of the Facility for Electron Microscopy Research and also acknowledge Dr. Janusz Rak and Laura Montermini at the Montreal Children’s Hospital for the use of the Nanosight NS500.
Funding information
This study received financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Foundation for Innovation (CFI), Le Fonds de recherche du Québec – Nature et technologies (FRQNT), Environment and Climate Change Canada, McGill University, and the Walter C. Sumner Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 3407 kb)
Rights and permissions
About this article
Cite this article
Ghoshdastidar, A.J., Ramamurthy, J., Morissette, M. et al. Development of methodology to generate, measure, and characterize the chemical composition of oxidized mercury nanoparticles. Anal Bioanal Chem 412, 691–702 (2020). https://doi.org/10.1007/s00216-019-02279-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02279-y