Abstract
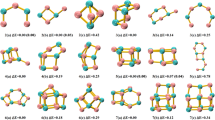
Herein the performance of a modification within the hybrid algorithm implemented in the AUTOMATON program is introduced and evaluated. For the creation of the initial population, AUTOMATON combines a probabilistic automata procedure with a genetic algorithm used to evolve this population. The proposed modification is addressed to efficiently identify the minimum energy structures of systems composed of more than one type of atom and with a low computational cost. The effectiveness of this approach is evaluated in the determination of the minimum energy structures of Be6B11−. The modification, aimed to explore the potential energy surface, consisted of filling the cells first with Be atoms in the process of creating the initial population. This order obeys the structural pattern established in the Be–B clusters reported to date. The results show that this variation not only identifies a more significant number of viable isomers but also to find a better putative global minimum than those previously reported in the literature. Therefore, it is recommended to be used as a complement to the standard searching process.


Similar content being viewed by others
References
Jortner J (1992) Clusters as a key to the understanding of properties as a function of size and dimensionality. In: Jena P, Khanna SN, Rao BK (eds) Physics and chemistry of finite systems: from clusters to crystals. Springer, Netherlands, pp 1–17
Alexandrova AN, Boldyrev AI, Zhai H-J, Wang L-S (2006) All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Coord Chem Rev 250:2811–2866. https://doi.org/10.1016/J.CCR.2006.03.032
Malinowski N, Schaber H, Bergmann T, Martin TP (1989) Electronic shell structure in NaO clusters. Solid State Commun 69:733–735. https://doi.org/10.1016/0038-1098(89)90820-X
Wade K (1976) Structural and bonding patterns in cluster chemistry. Adv Inorg Chem Radiochem 18:1–66. https://doi.org/10.1016/S0065-2792(08)60027-8
Wang L, Cheng H, Fan J (1995) Photoelectron spectroscopy of size-selected transition metal clusters: Fen − , n = 3–24. J Chem Phys 102:9480–9493. https://doi.org/10.1063/1.468817
León I, Yang Z, Liu H-T, Wang L-S (2014) The design and construction of a high-resolution velocity-map imaging apparatus for photoelectron spectroscopy studies of size-selected clusters. Rev Sci Instrum 85:083106. https://doi.org/10.1063/1.4891701
Li X, Kuznetsov AE, Zhang H-F, Boldyrev AI, Wang L-S (2001) Observation of all-metal aromatic molecules. Science 291(80):859. https://doi.org/10.1126/science.291.5505.859
Li J, Li X, Zhai H-J, Wang L-S (2003) Au20: a tetrahedral cluster. Science 299(80):864. https://doi.org/10.1126/science.1079879
Ji M, Gu X, Li X, Gong X, Li J, Wang L-S (2005) Experimental and theoretical investigation of the electronic and geometrical structures of the Au32 cluster. Angew Chemie Int Ed 44:7119–7123. https://doi.org/10.1002/anie.200502795
Baletto F, Ferrando R (2005) Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371–423. https://doi.org/10.1103/RevModPhys.77.371
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910. https://doi.org/10.1021/cr040090g
Metropolis N, Ulam S (1949) The monte carlo method. J Am Stat Assoc 44:335–341. https://doi.org/10.1080/01621459.1949.10483310
Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E (1953) Equation of state calculations by fast computing machines. J Chem Phys 21:1087–1092. https://doi.org/10.1063/1.1699114
Vanderbilt D, Louie SG (1984) A Monte carlo simulated annealing approach to optimization over continuous variables. J Comput Phys 56:259–271. https://doi.org/10.1016/0021-9991(84)90095-0
Van Laarhoven PJM, Aarts EHL (1987) Simulated annealing. In: Hwang CR (ed) Simulated annealing: theory and applications. Springer, Berlin, pp 7–15
Kirkpatrick S, Gelatt CD, Vecchi MP (1983) Optimization by simulated annealing. Science 220(80):671–680. https://doi.org/10.1126/science.220.4598.671
Hartke B (2002) Global geometry optimization of clusters using genetic algorithms. J Phys Chem 97:9973–9976. https://doi.org/10.1021/j100141a013
Hartke B (1995) Global geometry optimization of clusters using a growth strategy optimized by a genetic algorithm. Chem Phys Lett 240:560–565. https://doi.org/10.1016/0009-2614(95)00587-T
Rabanal-León WA, Tiznado W, Osorio E, Ferraro F (2018) Exploring the potential energy surface of small lead clusters using the gradient embedded genetic algorithm and an adequate treatment of relativistic effects. RSC Adv 8:145–152. https://doi.org/10.1039/C7RA11449D
Deaven DM, Ho KM (1995) Molecular geometry optimization with a genetic algorithm. Phys Rev Lett 75:288–291. https://doi.org/10.1103/PhysRevLett.75.288
Daven DM, Tit N, Morris JR, Ho KM (1996) Structural optimization of Lennard–Jones clusters by a genetic algorithm. Chem Phys Lett 256:195–200. https://doi.org/10.1016/0009-2614(96)00406-X
Alexandrova AN, Boldyrev AI, Fu Y-J, Yang X, Wang X-B, Wang L-S (2004) Structure of the NaxCl−x+1 (x = 1–4) clusters via ab initio genetic algorithm and photoelectron spectroscopy. J Chem Phys 121:5709–5719. https://doi.org/10.1063/1.1783276
Davis JBA, Shayeghi A, Horswell SL, Johnston RL (2015) The Birmingham parallel genetic algorithm and its application to the direct DFT global optimisation of IrN (N = 10–20) clusters. Nanoscale 7:14032–14038. https://doi.org/10.1039/C5NR03774C
Shayeghi A, Götz D, Davis JBA, Schäfer R, Johnston RL (2015) Pool-BCGA: a parallelised generation-free genetic algorithm for the ab initio global optimisation of nanoalloy clusters. Phys Chem Chem Phys 17:2104–2112. https://doi.org/10.1039/C4CP04323E
Johnston RL, Mortimer-Jones TV, Roberts C, Darby S, Manby FR (2002) Application of genetic algorithms in nanoscience: cluster geometry optimization BT. In: Cagnoni S, Gottlieb J, Hart E, Middendorf M, Raidl GR (eds) Applications of evolutionary computing. Springer, Berlin, pp 92–101
Vargas JA, Buendía F, Beltrán MR (2017) New AuN (N = 27–30) lowest energy clusters obtained by means of an improved DFT—genetic algorithm methodology. J Phys Chem C 121:10982–10991. https://doi.org/10.1021/acs.jpcc.6b12848
Kanters PFR, Donald KJ (2014) Cluster: searching for unique low energy minima of structures using a novel implementation of a genetic algorithm. J Chem Theory Comput 10:5729–5737. https://doi.org/10.1021/ct500744k
Eberhart R, Kennedy J (1995) A new optimizer using particle swarm theory. In: MHS’95. Proceedings of the sixth international symposium on micro machine and human science, pp 39–43
Poli R, Kennedy J, Blackwell T (2007) Particle swarm optimization. Swarm Intell 1:33–57. https://doi.org/10.1007/s11721-007-0002-0
Zhan Z, Zhang J, Li Y, Chung HS (2009) Adaptive particle swarm optimization. IEEE Trans Syst Man, Cybern Part B 39:1362–1381. https://doi.org/10.1109/TSMCB.2009.2015956
Call ST, Zubarev DY, Boldyrev AI (2007) Global minimum structure searches via particle swarm optimization. J Comput Chem 28:1177–1186. https://doi.org/10.1002/jcc.20621
Jana G, Mitra A, Pan S, Sural S, Chattaraj PK (2019) Modified particle swarm optimization algorithms for the generation of stable structures of carbon clusters, Cn (n = 3–6, 10). Front Chem 7:485
Li Z, Scheraga HA (1987) Monte Carlo-minimization approach to the multiple-minima problem in protein folding. Proc Natl Acad Sci 84:6611–6615. https://doi.org/10.1073/pnas.84.19.6611
Wales JD, Doye PKJ (1997) Global optimization by basin-hopping and the lowest energy structures of lennard-jones clusters containing up to 110 atoms. J Phys Chem A 101:5111–5116. https://doi.org/10.1021/jp970984n
White RP, Mayne HR (1998) An investigation of two approaches to basin hopping minimization for atomic and molecular clusters. Chem Phys Lett 289:463–468. https://doi.org/10.1016/S0009-2614(98)00431-X
Liberti L, Maculan N (2006) Global optimization: from theory to implementation. Springer, Berlin
Zhao Y, Chen X, Li J (2017) TGMin: a global-minimum structure search program based on a constrained basin-hopping algorithm. Nano Res 10:3407–3420. https://doi.org/10.1007/s12274-017-1553-z
Saunders M (1987) Stochastic exploration of molecular mechanics energy surfaces: hunting for the global minimum. J Am Chem Soc 109:3150–3152. https://doi.org/10.1021/ja00244a051
Bera PP, Schleyer PV, Schaefer HFR III (2007) Periodane: a wealth of structural possibilities revealed by the Kick procedure. Int J Quantum Chem 107:2220–2223. https://doi.org/10.1002/qua.21322
Averkiev B (2009) Geometry and electronic structure of doped clusters via the Coalescence Kick method. Utah State University, Logan
Addicoat MA, Metha GF (2009) Kick: constraining a stochastic search procedure with molecular fragments. J Comput Chem 30:57–64. https://doi.org/10.1002/jcc.21026
Cabellos JL, Ortiz-Chi F, Ramirez A, Merino G (2013) GLOMOS 1.0, Cinvestav, Mérida
Heiles S, Johnston RL (2013) Global optimization of clusters using electronic structure methods. Int J Quantum Chem 113:2091–2109. https://doi.org/10.1002/qua.24462
Zhang J, Dolg M (2016) Global optimization of clusters of rigid molecules using the artificial bee colony algorithm. Phys Chem Chem Phys 18:3003–3010. https://doi.org/10.1039/C5CP06313B
Jackson KA, Horoi M, Chaudhuri I, Frauenheim T, Shvartsburg AA (2004) Unraveling the shape transformation in silicon clusters. Phys Rev Lett 93:13401. https://doi.org/10.1103/PhysRevLett.93.013401
Avaltroni F, Corminboeuf C (2012) Identifying clusters as low-lying mimina—efficiency of stochastic and genetic algorithms using inexpensive electronic structure levels. J Comput Chem 33:502–508. https://doi.org/10.1002/jcc.22882
Zhao J, Shi R, Sai L, Huang X, Su Y (2016) Comprehensive genetic algorithm for ab initio global optimisation of clusters. Mol Simul 42:809–819. https://doi.org/10.1080/08927022.2015.1121386
Tiznado W, Perez-Peralta N, Islas R, Toro-Labbe A, Ugalde J, Merino G (2009) Designing 3-D molecular stars. J Am Chem Soc 131:9426–9431. https://doi.org/10.1021/ja903694d
Perez-Peralta N, Contreras M, Tiznado W, Stewart J, Donald KJ, Merino G (2011) Stabilizing carbon-lithium stars. Phys Chem Chem Phys 13:12975–12980. https://doi.org/10.1039/C1CP21061K
Torres-Vega JJ, Vásquez-Espinal A, Beltran MJ, Ruiz L, Islas R, Tiznado W (2015) Li7(BH)+5 : a new thermodynamically favored star-shaped molecule. Phys Chem Chem Phys 17:19602–19606. https://doi.org/10.1039/c5cp02006a
Contreras M, Osorio E, Ferraro F, Puga G, Donald KJ, Harrison JG, Merino G, Tiznado W (2013) Isomerization energy decomposition analysis for highly ionic systems: case study of starlike E5Li7+ clusters. Chem Eur J 19:2305–2310. https://doi.org/10.1002/chem.201203329
Vásquez-Espinal A, Palacio-Rodríguez K, Ravell E, Orozco-Ic M, Barroso J, Pan S, Tiznado W, Merino G (2018) E5M7+ (E = C–Pb, M = Li–Cs): a source of viable star-shaped clusters. Chem Asian J 13:1751–1755. https://doi.org/10.1002/asia.201800654
Yañez O, Garcia V, Garza J, Orellana W, Vásquez-Espinal A, Tiznado W (2019) (Li6Si5)2–5: the smallest cluster-assembled materials based on aromatic Si56− rings. Chem Eur J 25:2467–2471. https://doi.org/10.1002/chem.201805677
Vassilev-Galindo V, Pan S, Donald KJ, Merino G (2018) Planar pentacoordinate carbons. Nat Rev Chem 2:114
Ravell E, Jalife S, Barroso J, Orozco-Ic M, Hernández-Juárez G, Ortiz-Chi F, Pan S, Cabellos JL, Merino G (2018) Structure and bonding in CE5− (E = Al–Tl) clusters: planar tetracoordinate carbon versus pentacoordinate carbon. Chem Asian J 13:1467–1473. https://doi.org/10.1002/asia.201800261
Yañez O, Vasquez-Espinal A, Pino-Rios R, Ferraro F, Pan S, Osorio E, Merino G, Tiznado W (2017) Exploiting electronic strategies to stabilize a planar tetracoordinate carbon in cyclic aromatic hydrocarbons. Chem Commun 53:12112–12115. https://doi.org/10.1039/C7CC06248F
Yañez O, Vásquez-Espinal A, Báez-Grez R, Rabanal-León WA, Osorio E, Ruiz L, Tiznado W (2019) Carbon rings decorated with group 14 elements: new aromatic clusters containing planar tetracoordinate carbon. New J Chem 43:6781–6785. https://doi.org/10.1039/C9NJ01022J
García J-J, Hernández-Esparza R, Vargas R, Tiznado W, Garza J (2019) Formation of small clusters of NaCl dihydrate in the gas phase. New J Chem 43:4342–4348. https://doi.org/10.1039/C8NJ06315J
Fuentealba P, Cardenas C, Pino-Rios R, Tiznado W (2016) Topological analysis of the fukui function BT. In: Chauvin R, Lepetit C, Silvi B, Alikhani E (eds) Applications of topological methods in molecular chemistry. Springer, Cham, pp 227–241
Vásquez-Espinal A, Torres-Vega JJ, Alvarez-Thon L, Fuentealba P, Islas R, Tiznado W (2016) Boron avoids cycloalkane-like structures in the LinBnH2n series. New J Chem 40:2007–2013. https://doi.org/10.1039/c5nj02051d
Mondal S, Cabellos JL, Pan S, Osorio E, Torres-Vega JJ, Tiznado W, Restrepo A, Merino G (2016) 10-π-Electron arenes à la carte: structure and bonding of the [E–(CnHn)–E]n−6 (E = Ca, Sr, Ba; n = 6–8) complexes. Phys Chem Chem Phys 18:11909–11918. https://doi.org/10.1039/C6CP00671J
Dong X, Jalife S, Vásquez-Espinal A, Barroso J, Orozco-Ic M, Ravell E, Cabellos JL, Liang WY, Cui ZH, Merino G (2019) Li2B24: the simplest combination for a three-ring boron tube. Nanoscale 11:2143–2147. https://doi.org/10.1039/c8nr09173k
Liang W, Barroso J, Jalife S, Orozco-Ic M, Zarate X, Dong X, Cui Z-H, Merino G (2019) B10M2 (M = Rh, Ir): finally a stable boron-based icosahedral cluster. Chem Commun 55:7490–7493. https://doi.org/10.1039/C9CC03732B
Guo J-C, Feng L-Y, Wang Y-J, Jalife S, Vásquez-Espinal A, Cabellos JL, Pan S, Merino G, Zhai H-J (2017) Coaxial triple-layered versus helical Be6B11− clusters: dual structural fluxionality and multifold aromaticity. Angew Chem Int Ed 56:10174–10177. https://doi.org/10.1002/anie.201703979
Dong X, Jalife S, Vásquez-Espinal A, Ravell E, Pan S, Cabellos JL, Liang WY, Cui ZH, Merino G (2018) Li2B12 and Li3B12: prediction of the smallest tubular and cage-like boron structures. Angew Chem Int Ed 57:4627–4631. https://doi.org/10.1002/anie.201800976
Yanez O, Báez-Grez R, Inostroza D, Rabanal-León WA, Pino-Rios R, Garza J, Tiznado W (2019) AUTOMATON: a program that combines a probabilistic cellular automata and a genetic algorithm for global minimum search of clusters and molecules. J Chem Theory Comput 15:1463–1475. https://doi.org/10.1021/acs.jctc.8b00772
Fernández R, Louis P-Y, Nardi FR (2018) Overview: PCA models and issues. In: Probabilistic cellular automata. Springer, pp 1–30
Zhai H-J, Alexandrova AN, Birch KA, Boldyrev AI, Wang L-S (2003) Hepta- and octacoordinate boron in molecular wheels of eight- and nine-atom boron clusters: observation and confirmation. Angew Chem Int Ed 42:6004–6008. https://doi.org/10.1002/anie.200351874
Huang W, Sergeeva AP, Zhai H-J, Averkiev BB, Wang L-S, Boldyrev AI (2010) A concentric planar doubly π-aromatic B19− cluster. Nat Chem 2:202–206. https://doi.org/10.1038/nchem.534
Averkiev BB, Zubarev DY, Wang L-M, Huang W, Wang L-S, Boldyrev AI (2008) Carbon avoids hypercoordination in CB6−, CB62−, and C2B5− planar carbon − boron clusters. J Am Chem Soc 130:9248–9250. https://doi.org/10.1021/ja801211p
Romanescu C, Galeev TR, Li W-L, Boldyrev AI, Wang L-S (2011) Aromatic metal-centered monocyclic boron rings: co©B8− and Ru©B9−. Angew Chemie Int Ed 50:9334–9337. https://doi.org/10.1002/anie.201104166
Báez-Grez R, Garza J, Vásquez-Espinal A, Osorio E, Rabanal-León WA, Yañez O, Tiznado W (2019) Exploring the potential energy surface of trimetallic deltahedral zintl ions: lowest-energy [Sn6Ge2Bi]3– and [(Sn6Ge2Bi)2]4– structures. Inorg Chem 58:10057–10064. https://doi.org/10.1021/acs.inorgchem.9b01206
Grande-Aztatzi R, Martínez-Alanis PR, Cabellos JL, Osorio E, Martínez A, Merino G (2014) Structural evolution of small gold clusters doped by one and two boron atoms. J Comput Chem 35:2288–2296. https://doi.org/10.1002/jcc.23748
Ramirez-Manzanares A, Peña J, Azpiroz JM, Merino G (2015) A hierarchical algorithm for molecular similarity (H-FORMS). J Comput Chem 36:1456–1466. https://doi.org/10.1002/jcc.23947
Feng L-Y, Guo J-C, Li P-F, Zhai H-J (2018) Boron-based binary Be6B102− cluster: three-layered aromatic sandwich, electronic transmutation, and dynamic structural fluxionality. Phys Chem Chem Phys 20:22719–22729. https://doi.org/10.1039/C8CP04332A
Kang D, Sun W, Shi H, Lu C, Kuang X, Chen B, Xia X, Maroulis G (2019) Probing the structure and electronic properties of beryllium doped boron clusters: a planar BeB16− cluster motif for metallo-borophene. Sci Rep 9:14367. https://doi.org/10.1038/s41598-019-50905-7
Wang Y-J, Miao C-Q, Xie J-J, Wei Y-R, Ren G-M (2019) Be2B6 and Be2B7+: two double aromatic inverse sandwich complexes with spin-triplet ground state. New J Chem 43:15979–15982. https://doi.org/10.1039/C9NJ02819F
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170
Bergner A, Dolg M, Küchle W, Stoll H, Preuss H (1993) Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol Phys 80:1431–1441. https://doi.org/10.1080/00268979300103121
Igel-Mann G, Stoll H, Preuss H (1988) Pseudopotentials for main group elements (IIIa through VIIa). Mol Phys 65:1321–1328. https://doi.org/10.1080/00268978800101811
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/B508541A
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2013) Gaussian, Inc., Wallingford CT
CYLview, 1.0b; Legault, C. Y., Université de Sherbrooke, 2009 (http://www.cylview.org)
Jiménez-Halla JOC, Islas R, Heine T, Merino G (2010) B19−: an aromatic wankel motor. Angew Chem Int Ed 49:5668–5671. https://doi.org/10.1002/anie.201001275
Sergeeva AP, Popov IA, Piazza ZA, Li W-L, Romanescu C, Wang L-S, Boldyrev AI (2014) Understanding boron through size-selected clusters: structure, chemical bonding, and fluxionality. Acc Chem Res 47:1349–1358. https://doi.org/10.1021/ar400310g
Cervantes-Navarro F, Martínez-Guajardo G, Osorio E, Moreno D, Tiznado W, Islas R, Donald KJ, Merino G (2014) Stop rotating! One substitution halts the B19− motor. Chem Commun 50:10680–10682. https://doi.org/10.1039/C4CC03698K
Martínez-Guajardo G, Sergeeva AP, Boldyrev AI, Heine T, Ugalde JM, Merino G (2011) Unravelling phenomenon of internal rotation in B13+ through chemical bonding analysis. Chem Commun 47:6242–6244. https://doi.org/10.1039/C1CC10821B
Merino G, Heine T (2012) And yet it rotates: the starter for a molecular wankel motor. Angew Chem Int Ed 51:10226–10227. https://doi.org/10.1002/anie.201206188
Moreno D, Pan S, Zeonjuk LL, Islas R, Osorio E, Martínez-Guajardo G, Chattaraj PK, Heine T, Merino G (2014) B182−: a quasi-planar bowl member of the Wankel motor family. Chem Commun 50:8140–8143. https://doi.org/10.1039/C4CC02225D
Jalife S, Liu L, Pan S, Cabellos JL, Osorio E, Lu C, Heine T, Donald KJ, Merino G (2016) Dynamical behavior of boron clusters. Nanoscale 8:17639–17644. https://doi.org/10.1039/C6NR06383G
Pan S, Barroso J, Jalife S, Heine T, Asmis KR, Merino G (2019) Fluxional boron clusters: from theory to reality. Acc Chem Res 52:2732–2744. https://doi.org/10.1021/acs.accounts.9b00336
Acknowledgements
The authors are grateful for financial support from Fondecyt Grant 1181165. The authors are thankful for the facilities provided by the Laboratorio de Supercómputo y Visualización en Paralelo at Universidad Autónoma Metropolitana-Iztapalapa. The work in Mérida was supported by Conacyt (Grant CB–2015–252356) and Cinvestav (Grant SEP-Cinvestav-2018-57). J. B. thanks Conacyt for his Ph. D. fellowship. D. I. thanks Conicyt for his Ph.D fellowship (CONICYT PFCHA/BECAS DOCTORADO NACIONAL/2019−21190427).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles derived from the Chemical Concepts from Theory and Computation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yañez, O., Inostroza, D., Usuga-Acevedo, B. et al. Evaluation of restricted probabilistic cellular automata on the exploration of the potential energy surface of Be6B11−. Theor Chem Acc 139, 41 (2020). https://doi.org/10.1007/s00214-020-2548-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-2548-5