Abstract
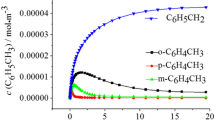
This work reports density functional and composite model chemistry calculations performed on the reactions of toluene with the hydroxyl radical. Both the experimentally observed H-abstraction from the methyl group and possible OH-additions to the phenyl ring were investigated. Reaction enthalpies and barrier heights suggest that H-abstraction is more favorable than OH-addition to the ring. The calculated reaction rates at room temperature and the radical-intermediate product fractions support this view. At first sight, this might seem to disagree with the fact that, under most experimental conditions, cresols are observed in a larger concentration than benzaldehyde. Since the accepted mechanism for benzaldehyde formation involves H-abstraction, a contradiction arises that calls for a more elaborate explanation. In this first exploratory study, we provide evidence that support the preference of H-abstraction over OH-addition and present an alternative mechanism which shows that cresols can be actually produced also through H-abstraction and not only from OH-addition, thus justifying the larger proportion of cresols than benzaldehyde among the products.






Similar content being viewed by others
References
National Academies of Sciences, Engineering, and Medicine (2016) The future of atmospheric chemistry research: remembering yesterday, understanding today, anticipating tomorrow. The National Academies Press, Washington, DC
Brasseur GP, Jacob DJ (2017) Modeling of atmospheric chemistry. CUP, Cambridge, UK
Akimoto H (2016) Atmospheric reaction chemistry. Springer, Tokyo
Atkinson R (1990) Gas-phase tropospheric chemistry of organic compounds: a review. Atmos Environ 24A:1–41
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem Rev 85:69–201
Yang Y, Shao M, Wang X, Nolscher AC, Kessel S, Guenther A, Williams J (2016) Towards a quantitative understanding of total OH reactivity: a review. Atmos Environ 134:147–161
Nayebzadeh M, Vahedpour M (2017) A review on reactions of polycyclic aromatic hydrocarbons with the most abundant atmospheric chemical fragments: theoretical and experimental data. Prof React Kinet Mech 42:201–220
Calvert JG, Atkinson R, Becker KH, Kamens RM, Seinfeld JH, Wallington TH, Yarwood G (2002) The mechanisms of atmospheric oxidation of the aromatic hydrocarbons. Oxford University Press, Oxford
Whitten GZ, Heo G, Kimura Y, McDonald-Buller E, Allen DT, Carter WPL, Yarwood G (2010) A new condensed toluene mechanism for carbon bond: CB05-TU. Atmos Environ 44:5346–5355
Davis DD, Bollinger W, Fischer S (1975) A kinetics study of the reaction of the OH free radical with aromatic compounds. 1. Absolute rate constants for reaction with benzene and toluene at 300°K. J Phys Chem 79:293–294
Perry RA, Atkinson R, Pitts JN Jr. (1977) Kinetics and mechanism of the gas phase reaction of hydroxyl radicals with aromatic hydrocarbons over the temperature range 296-473 K. J Phys Chem 81:296–304
Doyle GJ, Lloyd AC, Darnall KR, Winer AM, Pitts JN Jr. (1975) Gas phase kinetic study of relative rates of reaction of selected aromatic compounds with hydroxyl radicals in an environmental chamber. Environ Sci Tech 9:237–241
Hansen DA, Atkinson R, Pitts JN Jr. (1975) Rate constants for the reaction of OH radicals with a series of aromatic hydrocarbons. J Phys Chem 79:1763–1766
Tully FP, Ravishankara AR, Thompson RL, Nicovich JM, Shah RC, Kreutter NM, Wine PH (1981) Kinetics of the reactions of hydroxyl radical with benzene and toluene. J Phys Chem 85:2262–2269
Gery MW, Fox DL, Jeffries HE, Stockburger L, Weathers WS (1985) A Continuous Stirred Tank Reactor Investigation of the Gas-Phase Reaction of Hydroxyl Radicals and Toluene. Int J Chem Kinet 17:931–955
Hatipoglu A, Vione D, Yalcin Y, Minero C, Cinar Z (2010) Photo-oxidative degradation of toluene in aqueous media by hydroxyl radical. J Photochem Photobiol A: Chem 215:59–68
Zhang N, Geronimo I, Paneth P, Schindelka J, Schaefer T, Herrmann H, Richnow HH (2016) Analyzing sites of OH radical attack (ring vs. side chain) in oxidation of substituted benzenes via dual stable isotope analysis (δ13C and δ2H). Sci Tot Environ 542:484–494
Uc VH, García-Cruz I, Hernández-Laguna A, Vivier-Bunge A (2000) New channels in the reaction mechanism of the atmospheric oxidation of toluene. J Phys Chem A 104:7847–7855
Murakami Y, Oguchi T, Hashimoto K, Nosaka Y (2007) Theoretical study of the benzyl + O2 reaction: kinetics, mechanism, and product branching ratios. J Phys Chem A 111:13200–13208
da Silva G, Handam MR, Bozzelli JW (2009) Oxidation of the benzyl radical: mechanism, thermochemistry, and kinetics for the reactions of benzyl hydroperoxide. J Chem Theory Comput 5:3185–3194
Ji Y, Zhao J, Terazono H, Misawa K, Levitt NP, Li Y, Lin Y, Peng Y, Wang Y, Duan L, Pan B, Zhang F, Feng X, An T, Marrero-Ortiz W, Secrest J, Zhang AL, Shibuya K, Molina MJ, Zhang R (2017) Reassesing the atmospheric oxidation mechanism of toluene. PNAS 114:8169–8174
Zhang RM, Truhlar DG, Xu X (2019) Kinetics of the toluene reaction with OH radical. Research 2019:19
Möller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Pople JA, Nesbet RK (1954) Self-consistent orbitals for radicals. J Chem Phys 22:571–572
Dunning TH Jr. (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Montgomery JA Jr., Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822–2827
Montgomery JA Jr., Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory. J Chem Phys 126:084108
Adler TB, Knizia G, Werner HW (2007) A simple and efficient CCSD(T)-F12 approximation. J Chem Phys 127:221106
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, revision D.01. Gaussian Inc., Wallingford
Werner HJ, Knowles PJ, Knizia G et al (2012) Molpro, version 2012.1. University of Birmingham, Birmingham, UK
Uc VH, Álvarez-Idaboy JR, Galano A, García-Cruz I, Vivier-Bunge A (2006) Theoretical determination of the rate constant for OH hydrogen abstraction from toluene. J Phys Chem A 110:10155–10162
Ventura ON, Kieninger M, Salta Z, Kosmas AM, Barone V (2019) Enthalpies of formation of the benzyloxyl, benzylperoxyl, hydroxyphenyl radicals and related species on the potential energy surface for the reaction of toluene with the hydroxyl radical. Theor Chem Acc 138:115
Knispel R, Koch R, Siese M, Zetzch C (1990) Adduct formation of OH radicals with benzene, toluene, and phenol and consecutive reactions of the adducts with NOx and O2. Ber Bunsenges Phys Chem 94:1375–1379
Acknowledgements
This work has been supported by the Italian MIUR (PRIN 2017, project “Physico-chemical Heuristic Approaches: Nanoscale Theory Of Molecular Spectroscopy, PHANTOMS”, prot. 2017A4XRCA). The SMART@SNS Laboratory (http://smart.sns.it) is acknowledged for providing high-performance computer facilities. We gratefully acknowledge the financial contribution for making this study provided by Pedeciba, CSIC (UdelaR) and ANII. Some of the calculations reported in this paper were performed in ClusterUY, a newly installed platform for high-performance scientific computing at the National Supercomputing Center, Uruguay.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“Festschrift in honor of Prof. Fernando R. Ornellas” Guest Edited by Adélia Justino Aguiar Aquino, Antonio Gustavo Sampaio de Oliveira Filho & Francisco Bolivar Correto Machado.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salta, Z., Kosmas, A.M., Segovia, M.E. et al. A reinvestigation of the deceptively simple reaction of toluene with OH, and the fate of the benzyl radical: a combined thermodynamic and kinetic study on the competition between OH-addition and H-abstraction reactions. Theor Chem Acc 139, 112 (2020). https://doi.org/10.1007/s00214-020-02626-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02626-8