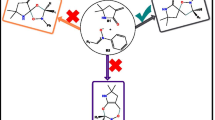
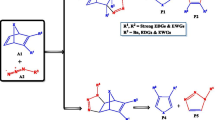
Abstract
1,3-Dipolar cycloaddition of nitrones to oxanorbornadienes is an important method for the enantioselective synthesis of highly substituted 5-membered heterocycles such as furans and isoxazolidines, which have high utility in the chemical and pharmaceutical industries. The mechanism of the reaction and the effects of substituents on the (3 + 2) cycloaddition reactions (32CA) of C,N-dialkyl nitrones with a series of substituted oxanorbornadienes have been studied with focus on the site-selectivity (attack on the more substituted double bond of the oxanorbornadiene derivatives versus attack on the less substituted double bond), enantioselectivity and stereo-selectivity using density functional theory calculations at the M06/6-311++G(d,p) of theory. The results showed that the addition step to form the bicyclic isoxazolidines cycloadducts has generally low barriers compared to the cycloreversion step which converts the cycloadducts into furans and monocyclic isoxazolidines. Generally, electron-withdrawing substituents favour the nitrone attack on the highly substituted double bond, while electron-donating substituents favour the attack on less substituted double bond. The R enantiomers are generally favoured over the S enantiomers, and exo stereo-isomers are generally favoured over the endo stereo-isomers, irrespective of substituents.










Similar content being viewed by others
References
Wanapun D, Van KA, Mosey NJ, Kerr MA, Woo TK (2005) The mechanism of 1,3-dipolar cycloaddition reactions of cyclopropanes and nitrones A theoretical study. Can J Chem. https://doi.org/10.1139/V05-182
Nie X, Lu C, Chen Z, Yang G, Nie J (2014) Enantioselective 1,3-dipolar cycloadditions of nitrones with unsaturated aldehydes promoted by a recyclable tetraarylphosphonium supported imidazolidinone catalyst. J Mol Catal A Chem 393:171–174. https://doi.org/10.1016/j.molcata.2014.06.015
Hashimoto T, Maruoka K (2015) Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem Rev 115:5366–5412. https://doi.org/10.1021/cr5007182
Alcaide B, Almendros P, Alonso JM, Aly MF, Pardo C, Sáez E, Torres MR (2002) Efficient entry to highly functionalized β-lactams by regio- and stereoselective 1,3-dipolar cycloaddition reaction of 2-azetidinone-tethered nitrones. J Org Chem 67:7004–7013. https://doi.org/10.1021/jo025924e
Cheviet T, Dujardin G, Parrot I, Martinez J, Mousseron M, De Montpellier U, Bataillon PE (2016) Isoxazolidine: a privileged scaffold for organic and medicinal chemistry. Chem Rev. https://doi.org/10.1021/acs.chemrev.6b00543
Aggarwal VK, Roseblade SJ, Barrell JK, Alexander R (2002) Highly diastereoselective nitrone cycloaddition onto a chiral ketene equivalent: asymmetric synthesis of cispentacin. Org Lett 4:1227–1229. https://doi.org/10.1021/ol025665f
Nacereddine AK, Yahia W, Bouacha S, Djerourou A (2010) A theoretical investigation of the regio- and stereoselectivities of the 1,3-dipolar cycloaddition of C-diethoxyphosphoryl-N-methylnitrone with substituted alkenes. Tetrahedron Lett 51:2617–2621. https://doi.org/10.1016/j.tetlet.2010.03.025
Mandal S, Maiti KK, Banerji A, Prangé T, Neuman A, Acharjee N (2018) Experimental and DFT studies for substituent effects on cycloadditions of C,N-disubstituted nitrones to cinnamoyl piperidine. Ind J Chem 57:108–119
Maiuolo L, De Nino A (2015) Synthesis of isoxazolidines by 1,3-dipolar cycloaddition: recent advances. Targets Heterocycl Syst 19:299–345
Meng L, Wang SC, Fettinger JC, Kurth MJ, Tantillo DJ (2009) Controlling selectivity for cycloadditions of nitrones and alkenes tethered by benzimidazoles: combining experiment and theory. Eur J Org Chem. https://doi.org/10.1002/ejoc.200801211
Gothelf KV, Jørgensen KA (1998) Asymmetric 1,3-dipolar cycloaddition reactions. Chem Rev. https://doi.org/10.1021/CR970324E
Cristina D, De Amici M, De Micheli C, Gandolfi R (1981) Site selectivity in the reactions of 1,3-dipoles with norbornadiene derivatives. Tetrahedron 37:1349–1357. https://doi.org/10.1016/S0040-4020(01)92451-2
Wavefunction, Inc. (2013) Spartan’14. Wavefunction Inc, Irvine
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, revision A.02. Gaussian Inc., Wallingford
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Acc Chem Res 41(2):157–167. https://doi.org/10.1021/ar700111a
Pieniazek SN, Houk KN (2006) The origin of the halogen effect on reactivity and reversibility of Diels–Alder cycloadditions involving furan. Ang Chem Int Ed 45(9):1442–1445. https://doi.org/10.1002/anie.200502677
Paton RS, Mackey JL, Kim WH, Lee JH, Danishefsky SJ, Houk KN (2010) Origins of stereoselectivity in the trans Diels–Alder paradigm. J Am Chem Soc 132(27):9335–9340. https://doi.org/10.1021/ja1009162
Paton RS, Steinhardt SE, Vanderwal CD, Houk KN (2011) Unraveling the mechanism of cascade reactions of zincke aldehydes. J Am Chem Soc 133:3895–3905. https://doi.org/10.1021/ja107988b
Wheeler SE, Moran A, Pieniazek SN, Houk KN (2009) Accurate reaction enthalpies and sources of error in DFT Thermochemistry for aldol, Mannich, and α-aminoxylation reactions. J Phys Chem A 113:10376–10384. https://doi.org/10.1021/jp9058565
Clark M, Cramer RD, Van Opdenbosch N (1989) Validation of the general purpose Tripos 5.2 force field. J Comput Chem 10(8):982–1012. https://doi.org/10.1002/jcc.540100804
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105(8):2999–3094. https://doi.org/10.1021/CR9904009
Opoku E, Tia R, Adei E (2019) Quantum chemical studies on the mechanistic aspects of tandem sequential cycloaddition reactions of cyclooctatetraene with ester and nitrones. J Mol Graph Model 92:17–31. https://doi.org/10.1016/J.JMGM.2019.06.019
Arhin G, Adams AH, Opoku E, Tia R, Adei E (2019) 1,3-Dipolar cycloaddition reactions of selected 1,3-dipoles with 7-isopropylidenenorbornadiene and follow-up thermolytic cleavage: a computational study. J Mol Graph Model 92:267–279. https://doi.org/10.1016/j.jmgm.2019.08.004
Opoku E, Tia R, Adei E (2019) DFT mechanistic study on tandem sequential [4 + 2]/[3 + 2] addition reaction of cyclooctatetraene with functionalized acetylenes and nitrile imines. J Phys Org Chem. https://doi.org/10.1002/poc.3992
Roland D, Haleegoah JN, Opoku E, Tia R, Adei E (2019) Mechanistic studies on tandem cascade [4 + 2]/[3 + 2] cycloaddition of 1,3,4-oxadiazoles with olefins. J Mol Graph Model 93:107452. https://doi.org/10.1016/j.jmgm.2019.107452
Opoku E, Tia R, Adei E (2019) Computational studies on [4 + 2]/[3 + 2] tandem sequential cycloaddition reactions of functionalized acetylenes with cyclopentadiene and diazoalkane for the formation of norbornene pyrazolines. J Mol Model 25:168. https://doi.org/10.1007/s00894-019-4056-x
Opoku E, Tia R, Adei E (2016) [3 + 2] Versus [2 + 2] addition: a density functional theory study on the mechanistic aspects of transition metal-assisted formation of 1, 2-dinitrosoalkanes. J Chem. https://doi.org/10.1155/2016/4538696
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121(9):1922–1924. https://doi.org/10.1021/ja983494x
Domingo LR, Chamorro E, Pérez P (2008) An understanding of the electrophilic/nucleophilic behavior of electro-deficient 2,3-disubstituted 1,3-butadienes in polar Diels–Alder reactions. A density functional theory study. J Phys Chem A 112(17):4046–4053. https://doi.org/10.1021/jp711704m
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions: a theoretical study. J Org Chem 73:4615–4624. https://doi.org/10.1021/jo800572a
Domingo LR, José AM, Pérez P, Contreras R (2002) Quantitative characterization of the local electrophilicity of organic molecules. Understanding the regioselectivity on Diels–Alder reactions. J Phys Chem A 106(29):6871–6875. https://doi.org/10.1021/jp020715j
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1(1–6):104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494. https://doi.org/10.1039/c2ra22886f
Acknowledgements
The authors are very grateful to the National Council for Tertiary Education, Republic of Ghana, for a research Grant under the Teaching and Learning Innovation Fund (TALIF/KNUST/3/0008/2005), and to South Africa’s Centre for High Performance Computing for access to additional computing resource on the Lengau cluster.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest whatsoever regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Opoku, E., Arhin, G., Pipim, G.B. et al. Site-, enantio- and stereo-selectivities of the 1,3-dipolar cycloaddition reactions of oxanorbornadiene with C,N-disubstituted nitrones and dimethyl nitrilimines: a DFT mechanistic study. Theor Chem Acc 139, 16 (2020). https://doi.org/10.1007/s00214-019-2529-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2529-8