Abstract
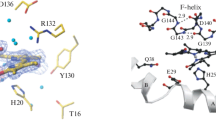
It has been shown that after production of oxophlorin, the first step of intermediate, both production of biliverdin and production of verdoheme occur simultaneously (Alavi et al. in Dalton Trans 47:8283–8291, 2018). So the mechanism that converts biliverdin into verdoheme is the subject of some controversy. The detailed conversion of verdoheme to biliverdin was demonstrated before by the Jerusalem group, using combined quantum mechanical and molecular mechanical (QM/MM) calculations. Conversion of iron biliverdin to iron verdoheme in the presence of H+ was investigated using the B3LYP method and the def2-QZVP basis set, considering dispersion effects with the DFT-D3 approach, obtaining accurate energies with large QM regions of almost 1000 atoms. Two spin states, singlet and triplet, were considered for the conversion of biliverdin to verdoheme. The reactant and product are triplet and singlet in their ground states, respectively. The potential energy surface suggests that a spin inversion takes place during the course of reaction after TS2. The ring closing process is exothermic by 5.8 kcal/mol with a kinetic barrier of 16.5 kcal/mol. The activation barrier for removing OH from the ring to produce iron verdoheme is estimated to be 23.2 kcal/mol.





Similar content being viewed by others
References
Alavi FS, Gheidi M, Zahedi M, Safari N, Ryde U (2018) A novel mechanism of heme degradation to biliverdin studied by QM/MM and QM calculations. Dalton Trans 47:8283–8291
Liu Y, Moënne-Loccoz P, Loehr TM, De Montellano PRO (1997) Heme oxygenase-1, intermediates in verdoheme formation and the requirement for reduction equivalents. J Biol Chem 272(11):6909–6917
Yoshida T, Noguchi M, Kikuchi G (1980) Oxygenated form of heme. Heme oxygenase complex and requirement for second electron to initiate heme degradation from the oxygenated complex. J Biol Chem 255(10):4418–4420
De Montellanoa PRO, Auclairb K (2003) Heme oxygenase structure and mechanism. In: Kadish K, Smith K, Guilard R (eds) The porphyrin handbook. Elsevier, Amsterdam, pp 183–210
Gheidi M, Safari N, Zahedi M (2014) Chameleonic nature of hydroxyheme in heme oxygenase and its reactivity: a density functional theory study. Inorg Chem 53(6):2766–2775
Alavi FS, Zahedi M, Safari N, Ryde U (2017) QM/MM study of the conversion of oxophlorin into verdoheme by heme oxygenase. J Phys Chem B 121(51):11427–11436
Gheidi M, Safari N, Zahedi M (2017) Density functional theory studies on the conversion of hydroxyheme to iron-verdoheme in the presence of dioxygen. Dalton Trans 46(7):2146–2158
Kirchner B, Wennmohs F, Ye S, Neese F (2007) Theoretical bioinorganic chemistry: the electronic structure makes a difference. Curr Opin Chem Biol 11(2):134–141
Hu H, Yang W (2008) Free energies of chemical reactions in solution and in enzymes with ab initio quantum mechanics/molecular mechanics methods. Annu Rev Phys Chem 59:573–601
Kamerlin SC, Haranczyk M, Warshel A (2008) Progress in ab initio QM/MM free-energy simulations of electrostatic energies in proteins: accelerated QM/MM studies of p K a, redox reactions and solvation free energies. J Phys Chem B 113(5):1253–1272
Neese F (2009) Prediction of molecular properties and molecular spectroscopy with density functional theory: from fundamental theory to exchange-coupling. Coord Chem Rev 253(5–6):526–563
Senn HM, Thiel W (2009) QM/MM methods for biomolecular systems. Angew Chem Int Ed 48(7):1198–1229
Siegbahn PE, Himo F (2009) Recent developments of the quantum chemical cluster approach for modeling enzyme reactions. J Biol Inorg Chem 14(5):643–651
Lai W, Chen H, Matsui T, Omori K, Unno M, Ikeda-Saito M, Shaik S (2010) Enzymatic ring-opening mechanism of verdoheme by the heme oxygenase: a combined X-ray crystallography and QM/MM study. J Am Chem Soc 132(37):12960–12970
Lad L, Schuller DJ, Shimizu H, Friedman J, Li H, de Montellano PRO, Poulos TL (2003) Comparison of the heme-free and-bound crystal structures of human heme oxygenase-1. J Biol Chem 278(10):7834–7843
Ryde U (1996) The coordination of the catalytic zinc ion in alcohol dehydrogenase studied by combined quantum-chemical and molecular mechanics calculations. J Comput Aided Mol Des 10(2):153–164
Ryde U, Olsson MH (2001) Structure, strain, and reorganization energy of blue copper models in the protein. Int J Quantum Chem 81(5):335–347
Ryde U, Olsen L, Nilsson K (2002) Quantum chemical geometry optimizations in proteins using crystallographic raw data. J Comput Chem 23(11):1058–1070
Treutler O, Ahlrichs R (1995) Efficient molecular numerical integration schemes. J Chem Phys 102(1):346–354
Case DA, Darden T, Cheatham TE III, Simmerling C, Wang J, Duke RE, Luo R, Merz KM, Pearlman DA, Crowley M (2006) AMBER 9. University of California, San Francisco, p 45
Lecerof D, Fodje M, Hansson A, Hansson M, Al-Karadaghi S (2000) Structural and mechanistic basis of porphyrin metallation by ferrochelatase. J Mol Biol 297(1):221–232
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91(14):146401
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32(7):1456–1465
Eichkorn K, Treutler O, Oehm H, Häser M, Ahlrichs R (1995) Auxiliary basis sets to approximate Coulomb potentials. Chem Phys 240(4):283–290
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Hertwig RH, Koch W (1997) On the parameterization of the local correlation functional. What is Becke-3-LYP? Chem Phys Lett 268(5–6):345–351
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor Chem Acc 97(1–4):119–124
Hu L, Söderhjelm P, Ryde U (2011) On the convergence of QM/MM energies. J Chem Theory Comput 7(3):761–777
Sumner S, Söderhjelm P, Ryde U (2013) Effect of geometry optimizations on QM-cluster and QM/MM studies of reaction energies in proteins. J Chem Theory Comput 9(9):4205–4214
Green MT (1999) Evidence for sulfur-based radicals in thiolate compound I intermediates. J Am Chem Soc 121(34):7939–7940
Ghosh A, Wondimagegn T (2000) A theoretical study of axial tilting and equatorial asymmetry in metalloporphyrin–nitrosyl complexes. J Am Chem Soc 122(33):8101–8102
Kumar D, Hirao H, Que L, Shaik S (2005) Theoretical investigation of C–H hydroxylation by (N4Py) FeIV O2+ : an oxidant more powerful than P450? J Am Chem Soc 127(22):8026–8027
Blomberg MR, Borowski T, Himo F, Liao R-Z, Siegbahn PE (2014) Quantum chemical studies of mechanisms for metalloenzymes. Chem Rev 114(7):3601–3658
Ryde U (2007) Accurate metal-site structures in proteins obtained by combining experimental data and quantum chemistry. Dalton Trans 6:607–625
Delcey MG, Pierloot K, Phung QM, Vancoillie S, Lindh R, Ryde U (2014) Accurate calculations of geometries and singlet–triplet energy differences for active-site models of [NiFe] hydrogenase. Phys Chem Chem Phys 16(17):7927–7938
Dong G, Ryde U (2016) Protonation states of intermediates in the reaction mechanism of [NiFe] hydrogenase studied by computational methods. J Biol Inorg Chem 21(3):383–394
Li J-L, Mata RA, Ryde U (2013) Large density-functional and basis-set effects for the dmso reductase catalyzed oxo-transfer reaction. J Chem Theory Comput 9(3):1799–1807
Acknowledgements
This investigation has been supported by grants from the research council of Shahid Beheshti University and the Swedish research council (Project 2014-5540). The computations were performed on computer resources provided by the Swedish National Infrastructure for Computing (SNIC) at Lunarc at Lund University and HPC2N at Umeå University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alavi, F.S., Zahedi, M., Safari, N. et al. QM/MM study of the conversion of biliverdin into verdoheme by heme oxygenase. Theor Chem Acc 138, 72 (2019). https://doi.org/10.1007/s00214-019-2461-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2461-y