Abstract
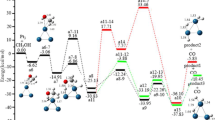
The density functional theory has been used to investigate the methanol adsorption, decomposition and oxidation as well as the water adsorption and decomposition on the clean and Pt-encapsulated Al12N12 cages. It is shown that platinum metal atom can be encapsulated within the Al12N12 cage, thus forming Pt-encapsulated Al12N12 cage electrocatalyst. According to the reaction results, CH3OH and H2O both prefer to be adsorbed on the Al atom top site. The O–H bond scission is the significantly more favorable reaction pathway than C–O bond scission on the cage surface. Furthermore, the structures of the intermediates, transition states and products, the corresponding energy barriers and reaction energies are confirmed. The results also show that the adsorption energies and energy barriers of CH3OH and H2O dissociation are cut down slightly with the aid of the platinum atom. Nevertheless, Pt-encapsulated Al12N12 cage makes the potential energy surface changes smoother than pure Al12N12 cage.









Similar content being viewed by others
References
Cheng FY, Chen J (2012) Metal-air batteries: from oxygen reduction electrochemistry to cathode catalysts. Chem Soc Rev 41:2172–2192
Dell RM, Rand DAJ (2001) Energy storage—a key technology for global energy sustainability. J Power Sources 100:2–17
Fabbri E, Pergolesi D, Traversa E (2010) Materials challenges toward proton-conducting oxide fuel cells: a critical review. Chem Soc Rev 39:4355–4369
Steele BCH, Heinzel A (2001) Materials for fuel-cell technologies. Nature 414:345–352
Yang J, Sudik A, Wolverton C, Siegel DJ (2010) High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Chem Soc Rev 39:656–675
Wang Y, Chen KS, Mishler J, Cho SC, Adroher XC (2011) A review of polymer electrolyte membrane fuel cells: technology, applications, and needs on fundamental research. Appl Energy 88:981–1007
Huber GW, Shabaker JW, Dumesic JA (2003) Raney Ni-Sn catalyst for H-2 production from biomass-derived hydrocarbons. Science 300:2075–2077
Cheng W-H (1995) Deactivation and regeneration of Cu/Cr based methanol decomposition catalysts. Appl Catal B Environ 7:127–136
Beden B, Léger J-M, Lamy C (1992) Electrocatalytic oxidation of oxygenated aliphatic organic compounds at noble metal electrodes, modern aspects of electrochemistry. Springer, New York, pp 97–264
Wang H, He CZ, Huai LY, Liu JY (2013) Decomposition and oxidation of methanol on Ir(111): a first-principles study. J Phys Chem C 117:4574–4584
Hamnett A (1997) Mechanism and electrocatalysis in the direct methanol fuel cell. Catal Today 38:445–457
Kandoi S, Greeley J, Sanchez-Castillo MA, Evans ST, Gokhale AA, Dumesic JA, Mavrikakis M (2006) Prediction of experimental methanol decomposition rates on platinum from first principles. Top Catal 37:17–28
Greeley J, Mavrikakis M (2004) Competitive paths for methanol decomposition on Pt(111). J Am Chem Soc 126:3910–3919
Franaszczuk K, Herrero E, Zelenay P, Wieckowski A, Wang J, Masel R (1992) A comparison of electrochemical and gas-phase decomposition of methanol on platinum surfaces. J Phys Chem 96:8509–8516
Mei DH, Xu LJ, Henkelman G (2009) Potential energy surface of methanol decomposition on Cu(110). J Phys Chem C 113:4522–4537
Gu XK, Li WX (2010) First-principles study on the origin of the different selectivities for methanol steam reforming on Cu(111) and Pd(111). J Phys Chem C 114:21539–21547
Esrafili MD, Nurazar R (2014) A density functional theory study on the adsorption and decomposition of methanol on B12N12 fullerene-like nanocage. Superlattice Microstruct 67:54–60
Esrafili MD, Nurazar R (2014) Potential of C-doped boron nitride fullerene as a catalyst for methanol dehydrogenation. Comput Mater Sci 92:172–177
Soleymanabadi H, Peyghan AA (2013) Decomposition of methanol on nanosized tube of magnesium oxide: a theoretical study. Comput Mater Sci 79:182–186
Esrafili MD, Nematollahi P, Nurazar R (2016) A density functional theory study on adsorption and decomposition of acetic acid over silicon carbide nanotubes. Synth Met 215:164–169
Burghaus U, Bye D, Cosert K, Goering J, Guerard A, Kadossov E, Lee E, Nadoyama Y, Richter N, Schaefer E, Smith J, Ulness D, Wymore B (2007) Methanol adsorption in carbon nanotubes. Chem Phys Lett 442:344–347
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: Buckminsterfullerene. Nature 318:162–163
Toth E, Bolskar RD, Borel A, Gonzalez G, Helm L, Merbach AE, Sitharaman B, Wilson LJ (2005) Water-soluble gadofullerenes: toward high-relaxivity, pH-responsive MRI contrast agents. J Am Chem Soc 127:799–805
Czochara R, Ziaja P, Piotrowski P, Pokrop R, Litwinienko G (2012) Fullerene C-60 as an inhibitor of high temperature lipid oxidation. Carbon 50:3943–3946
Gedikpınar M, Çavaş M, Alahmed ZA, Yakuphanoglu F (2013) Electronic properties of Al/p–Si/C70/Au MIS-type diode. Superlattice Microstruct 59:123–132
Beheshtian J, Behzadi H, Esrafili MD, Shirvani BB, Hadipour NL (2010) A computational study of water adsorption on boron nitride nanotube. Struct Chem 21:903–908
Esrafili MD, Behzadi H (2013) A DFT study on carbon-doping at different sites of (8,0) boron nitride nanotube. Struct Chem 24:573–581
Wang Q, Sun Q, Jena P, Kawazoe Y (2009) Potential of AlN nanostructures as hydrogen storage materials. ACS Nano 3:621–626
Wu HS, Zhang FQ, Xu XH, Zhang CJ, Jiao HJ (2003) Geometric and energetic aspects of aluminum nitride cages. J Phys Chem A 107:204–209
Esrafili MD, Nurazar R (2014) Efficient dehydrogenation of formic acid using Al12N12 nanocage: a DFT study. Superlattice Microstruct 75:17–26
Jiang Z, Fang T (2016) Dissociation mechanism of H2O on clean and oxygen-covered Cu(111) surfaces: a theoretical study. Vacuum 128:252–258
Niu M, Yu GT, Yang GH, Chen W, Zhao XG, Huang XR (2014) Doping the alkali atom: an effective strategy to improve the electronic and nonlinear optical properties of the inorganic Al12N12 nanocage. Inorg Chem 53:349–358
Xu HX, Zhang M, Chen YY, Liu HL, Xu KJ, Huang XR (1061) The catalytic activity of Pt6 M (M = Pt, Ru, Sn) cluster for methanol partial oxidation. Comput Theor Chem 2015:52–59
Esrafili MD, Nurazar R (2015) Metal-free decomposition of formic acid on pristine and carbon-doped boron nitride fullerene: a DFT study. J Cluster Sci 26:595–608
Zhang YH, Zheng XZ, Zhang SL, Huang SP, Wang P, Tian HP (2012) Bare and Ni decorated Al12N12 cage for hydrogen storage: a first-principles study. Int J Hydrog Energy 37:12411–12419
Solimannejad M, Kamalinahad S, Shakerzadeh E (2016) Selective detection of toxic cyanogen gas in the presence of O2, and H2O molecules using a AlN nanocluster. Phys Lett A 380:2854–2860
Gilani MA, Tabassum S, Gul U, Mahmood T, Alharthi AI, Alotaibi MA, Geesi M, Sheikh R, Ayub K (2018) Copper-doped Al12N12 nano-cages: potential candidates for nonlinear optical materials. Appl Phys A 124:14. https://doi.org/10.1007/s00339-017-1425-0
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09, revision D. 01. Gaussian Inc., Wallingford
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Shokuhi Rad A, Bagheri Novir S, Mohseni S, Ramezani Cherati N, Mirabi A (2017) A DFT study of O2 and Cl2 adsorption onto Al12N12 fullerene-like nanocluster. Heteroatom Chem 28:e21396
Tahmasebi E, Shakerzadeh E, Biglari Z (2016) Theoretical assessment of the electro-optical features of the group III nitrides (B12N12, Al12N12 and Ga12N12) and group IV carbides (C24, Si12C12 and Ge12C12) nanoclusters encapsulated with alkali metals (Li, Na and K). Appl Surf Sci 363:197–208
Tazikeh-Lemeski E (2017) Al12CN11 nano-cage sensitive to NH3 detection: a first-principles study. J Mol Struct 1135:166–173
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys 82:299–310
Soltani A, Raz SG, Taghartapeh MR, Moradi AV, Mehrabian RZ (2013) Ab initio study of the NO2 and SO2 adsorption on Al12N12 nano-cage sensitized with gallium and magnesium. Comput Mater Sci 79:795–803
Costales A, Blanco MA, Francisco E, Pendas AM, Pandey R (2006) First principles study of neutral and anionic (medium-size) aluminum nitride clusters: AlnNn, n = 7-16. J Phys Chem B 110:4092–4098
Shakerzadeh E, Barazesh N, Talebi SZ (2014) A comparative theoretical study on the structural, electronic and nonlinear optical features of B12N12 and Al12N12 nanoclusters with the groups III, IV and V dopants. Superlattice Microstruct 76:264–276
Zhou Y-H, Lv P-H, Wang G-C (2006) DFT studies of methanol decomposition on Ni(100) surface: compared with Ni(111) surface. J Mol Catal A Chem 258:203–215
Vo CT, Huynh LK, Hung JY, Jiang J-C (2013) Methanol adsorption and decomposition on ZnO(1010) surface: a density functional theory study. Appl Surf Sci 280:219–224
Shan J, Kleyn AW, Juurlink LB (2009) Identification of hydroxyl on Ni(111). ChemPhysChem 10:270–275
Bedürftig K, Völkening S, Wang Y, Wintterlin J, Jacobi K, Ertl G (1999) Vibrational and structural properties of OH adsorbed on Pt(111). J Chem Phys 111:11147–11154
Ma FF, Ma SH, Jiao ZY, Dai XQ (2016) Adsorption and decomposition of H2O on cobalt surfaces: a DFT study. Appl Surf Sci 384:10–17
Rui W, Wei F, Dandan Z, Huiling L, Huanyu Z, Xuri H (2017) Geometric stability of PtFe/PdFe embedded in graphene and catalytic activity for CO oxidation. Appl Organomet Chem 31:e3808
Acknowledgements
The authors thank the National Natural Science Foundation of China (Nos. 21573090, 21373099, 21403086) and Scientific Research Fund of Jilin Provincial Education Department (2015437) for financial support of this research and Science and technology research project of Jilin Provincial Department of education in 12th Five-Year Plan (No. 388[2011]).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, D., Feng, W., Liu, H. et al. Dissociation and oxidation mechanism of methanol on Al12N12 cage: a DFT study. Theor Chem Acc 137, 113 (2018). https://doi.org/10.1007/s00214-018-2292-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2292-2